Archived - Feed Labelling Regulatory Framework Proposal
This page has been archived
Information identified as archived is provided for reference, research or record-keeping purposes. It is not subject to the Government of Canada Web Standards and has not been altered or updated since it was archived. Please contact us to request a format other than those available.
This consultation closed 2013-12-20
Table of Contents
1 Purpose
This document outlines the principles, objectives and proposed direction of a modernized regulatory framework for livestock feed labels in Canada and seeks broad stakeholder comments and input on the regulatory proposal.
This proposal is one of the four modules that are being presented as part of the Feed Regulatory Renewal project. The other modules are:
- Feed ingredient assessment and authorization;
- Feed hazard identification and preventive controls; and
- Facility licensing and enforcement approaches.
2 Background
2.1 Change Management at the CFIA
The Canadian Food Inspection Agency (CFIA) has embarked on a comprehensive change agenda to strengthen its foundation of legislation, regulatory programs, and inspection delivery. These directions set the context for renewal of the Feeds Regulations (Regulations).
In December 2011, the CFIA initiated a systematic, multi-year review of its regulatory frameworks for food safety, plant health and animal health. Through this structured and comprehensive review, the Agency intends to update its regulatory frameworks to:
- reduce overlap and redundancy;
- increase responsiveness to industry changes;
- address gaps, weaknesses and inconsistencies; and
- provide clarity and flexibility to affected regulated parties.
The modernization of the Regulations has been identified as a short-term priority in the first three years of the modernization initiative.
The Agency will continue to explore opportunities for modernizing its legislative base as regulation modernization takes place. In the case of feeds this could mean seeking to update the Feeds Act (Act) with modern powers such as incorporation by reference (the inclusion of external documents into regulations) and novel approaches to feed regulation such as licensing facilities.
The Agency is engaged in developing a new inspection delivery model that is based on common inspection activities and standard processes for all of its inspection programs. This new model will be more focused on risk as the basis for prioritizing inspection activities. This new model, supported by a renewed training regime, is being pioneered for food inspection. It will be adapted as appropriate to help streamline and create efficiencies for other inspection activities including feed.
2.2 Feed Regulatory Modernization
The goal of modernizing the Regulations is to reduce compliance burden and support innovation while maintaining animal and human health, as well as environmental and economic sustainability.
The current feed regulatory framework is product-based including pre-market assessment and approval or registration of single feed ingredients and any mixed feeds (comprised of one or more ingredients) that do not meet exemption from registration criteria. In addition, the Regulations include requirements for labelling of feeds to compel accurate information on:
- the purpose of the ingredient or feed;
- the physical properties of the feed;
- the nutritional components;
- truthful claims; and
- information about its:
- nature;
- proper use; and
- any necessary details to ensure safe and effective use.
The Agency maintains post-market oversight of ingredients and feeds to further assure they are compliant with the composition, labelling and registration standards of these Regulations.
The modernization of the Regulations is being designed to benefit the collective Canadian feed industry which includes commercial feed manufacturers, retailers, importers, exporters, ingredient manufacturers, and farmers. As well as aligning with other international feed regulatory regimes, modernization also maintains the objective of enhancing animal health and food safety for the Canadian public.
2.3 Principles of CFIA Regulatory Modernization
The following principles guide the overall CFIA regulatory modernization process:
- protecting public safety and confidence in the inspection framework;
- enabling an environment of improved business opportunity and consumer choice by facilitating innovation and competitiveness;
- establishing clear policy objectives through consultation with partners - including industry, consumers, small business, other departments and provinces;
- finding the appropriate balance between administrative costs and benefits derived from regulatory intervention;
- developing consistent, transparent and, where appropriate, outcome-based regulations with performance measurements;
- recognizing the vital importance of science and risk management approaches in decision making; and
- aligning, to the extent possible, with international trading partner and provincial standards.
The ultimate objective of the review of the Regulations is to develop a modernized risk- and outcome-based regulatory framework for feeds that:
- supports fair and competitive trade in the market;
- minimizes regulatory burden; and
- protects animal health, the environment, and the food production continuum.
2.4 Consultation Process and the Role of this Proposal
Stakeholder consultation is an important component of regulatory modernization. The CFIA conducted several pre-consultation activities with stakeholders during 2012 to stimulate interest in a renewed feed regulatory framework, as well as identify current and future trends, opportunities, and challenges facing stakeholders as they might relate to feed production and use.
The Agency took a three-tiered approach to this pre-consultation. First, informal bilateral meetings were held with stakeholder groups. Second, a discussion paper and survey were made available on-line to provide all stakeholders with the opportunity to provide feedback. Finally, a two-day multi-stakeholder workshop was held in September 2012.
Following the September 2012 workshop there was recognition that further stakeholder consultation was needed. An industry and government "Feed Regulatory Steering Group" (FRSG) was formed in November 2012 with members representing the commercial feed manufacturers, major national livestock producer associations, the CFIA and Health Canada's Veterinary Drugs Directorate. Early on the FRSG determined that feed regulatory modernization was sufficiently complex that it should be broken down into the following components ("modules") for the further discussion:
- feed ingredient assessment and authorization;
- feed hazard identification and preventive controls (possibly including facility licensing);
- feed labelling; and
- facility licensing and enforcement approaches.
The information provided and gathered from these activities has formed the foundation of this proposal on feed labelling.
3 Current Framework - Feed Labelling
Currently, the labelling requirements for feeds are specified in the Feeds Regulations (Regulations) with a few additional labelling requirements, such as those specific to liquid feeds, elaborated in policy. There is a section of the Regulations that specifically addresses labelling, and more details are provided in the Regulations for specific feed types such as medicated feeds or single ingredient feeds.
3.1 Scope
This proposal addresses the regulation of feed labelling including:
- mandatory labelling requirements for all feed types;
- mandatory labelling requirements for specific feed types;
- voluntary labelling allowances; and
- information that will not be allowed on labels.
In the proposal below, in most cases, the current situation will be described for that particular topic, followed by any proposed changes.
In this proposal, labels will continue to be defined as they currently are in the Feeds Act:
"Label" includes any legend, word, mark, symbol or design applied to, included in, belonging to or accompanying any feed or package.
3.2 Objectives of Labelling
Risks associated with the representation and use of feed products can be mitigated by the use of labels. Labels can reduce the risks by providing:
- caution or warning statements which indicate a specific risk;
- directions for use which indicate how to safely use the product; and
- guaranteed analysis and lists of ingredients which allow the purchaser to know what is in the product and thus use it safely.
Labels play an important role in the safe and proper use of feeds. Proper labelling allows a purchaser and user of a feed to distinguish one product from another and provides information on what the feed is and how it is to be used. Products which are not labelled, or do not have the appropriate information on the label, may be unintentionally used in a manner that is not safe and causes an animal health, food safety or environmental hazard.
Regulation of labels also helps to assure a fair and level playing field. Internationally, guidance provided by the Codex Code of Practice on Good Animal Feeding - PDF (179 kb) (Code of Practice) includes labelling as one of the general principles and requirements, and the Code of Practice indicates that labels should be "clear and informative as to how the user should handle, store, and use feed and feed ingredients". This is consistent with international practices as all jurisdictions require some form of labelling for feed products.
3.3 Consultation
Aspects of labelling have been included in all of the consultation processes to date. Stakeholder feedback regarding labels has generally indicated that the current labelling requirements are appropriate, although there are some areas which require further discussion.
Labelling was addressed as part of the 2012 discussion paper and survey. The survey indicated general support regarding:
- the need for nutritional information on labels;
- labels should be truthful;
- lot numbers should be required; and
- additional label information should be allowed.
In addition, for those that filled out the more detailed survey questions regarding the Code of Practice, there was a high level of agreement that the following components should be part of the labelling framework:
- species;
- intended purpose;
- list of ingredients;
- contact information for manufacturer or registrant;
- directions for use; and
- lot identification.
There were also a few areas where there was a need for further discussion and elaboration. This included the presence of claims on labels, and foreign languages and information on labels.
Labelling was also part of the CFIA's September 2012 multi-stakeholder consultation workshop. At the workshop, the Code of Practice was used as the model for the discussion, and there was general consensus that the Code of Practice requirements were appropriate for feeds, were being applied internationally, and as such should be incorporated into the Regulations. However, the Code of Practice requirements are fairly general and the current Regulations include a number of other labelling requirements which were not addressed by the Code of Practice.
An industry and government FRSG was formed following the workshop, and decided that labelling was one of the modules to be addressed. A working meeting was held in May 2013 between the CFIA, Animal Nutrition Association of Canada (ANAC) and ANAC members who expressed an interest in participating in the discussion. This working meeting focused on the details concerning labelling, as it was recognized that there are a few specific issues that should be within the scope of a modernized regulatory framework for feeds. Feedback received on those issues has been considered in the drafting of this proposal.
4 Proposal
The Agency has settled on six general principles for labelling:
- labels must provide accurate information and not be misleading;
- labels must be clear and legible;
- labels must provide essential and useful information to permit safe and effective use of the product;
- labels should be appropriate for the product that they represent;
- label requirements should be flexible to allow for innovation and adaptation to new products; and
- accompanying materials are considered part of the label.
To assist in understanding the proposed labelling requirements, Annex 1 provides a sample label with the various requirements identified. The numbers on the label coordinate with the paragraph numbers below.
4.1 Mandatory Labelling Requirements
It is proposed that the following requirements for labels be mandatory for all feed and feed ingredients sold in Canada:
4.1.1 Feed Name (not to be confused with the Brand name)
The feed name should reflect the following components:
Purpose or Feed Type
The purpose for which a feed is intended shall be identified as part of the feed name. The purpose may be a complete feed, a supplement, a premix, antioxidant, colour, etc. This allows the purchaser to know how the feed is intended to be used.
Species or Category of Intended Livestock
The species or category of livestock for which the product is intended must be identified on the label as part of the feed name. If a product is intended for all species of livestock it does not need to indicate a species or it may indicate "for all livestock". This requirement is consistent internationally. As different species have different nutritional requirements this allows to the purchaser to purchase the correct formulation for their type of livestock.
As a new provision, to allow greater flexibility with products marketed in both Canada and the United States (U.S.), the purpose of the feed and species for which it is intended may appear as part of a purpose statement. For example, a product name may indicate "beef mineral" and a purpose statement would further elaborate "mineral for beef cattle on fescue pasture". The purpose statement would be optional, but may be used to meet the requirements of the feed name.
The inclusion of these two components allows the purchaser to recognize what the feed is and to differentiate the feed from others in the market. Feed names should be appropriate for the intended use of the feed and should not be misleading, for example, "Complete feed for Dairy Cattle", "Vitamin Supplement for Horses", "Micro Premix for all livestock". This is the current regulatory requirement for feed name and the proposal would maintain the status quo.
4.1.2 Manufacturer Contact Information (new)
Under the current Regulations feed labels must include "the name and address of the person who manufactured the feed or caused it to be manufactured". To improve clarity and usefulness, it is proposed that labels must specify the name and address of the actual place of manufacture. Additional names and addresses such as those of the head office for the company, importer or distributor may also be included on the label on a voluntary basis. The label must clearly indicate which address is the manufacturer of the feed. This requirement will improve traceability and will contribute to more timely response in a feed recall situation.
4.1.3 Registrant contact Information (new)
Under the current regulation all feeds which require registration must indicate the name and address of the registrant instead of the manufacturer. It is proposed that feeds which are registered must indicate the name and address of the registrant in addition to the name and address of the manufacturer. This will contribute to improved traceability and will provide the purchaser with useful information.
4.1.4 List of ingredients (new)
Under the current Regulations a label must have either the list of ingredients used in the manufacture of the feed, or a statement to the effect that the list may be obtained from the manufacturer. To be more consistent with the Code of Practice and other international jurisdictions and to provide purchasers of feeds with useful information, it is proposed that all feeds require the complete list of ingredients for that feed on the label. This will allow purchasers to:
- make informed decisions;
- prevent over supplementation of nutrients when multiple sources of an ingredient may be possible through greater clarity; and
- facilitate carry-over of warning, caution and safety statements.
Collective terms would be allowed for specific ingredient groups to facilitate least cost formulation of feeds and to be in line with the U.S. The actual list of ingredients must be provided, upon request, so that purchasers and government are able to know what ingredients are actually used in the feed.
4.1.5 Directions for Use
Under the current Regulations feed labels must include directions for use to permit the safe use of the feed. It is proposed that there are no changes to this requirement. This is consistent internationally and allows the purchaser to know how to use the feed safely and for its intended purpose. Without adequate directions for use a feed could be unintentionally misused resulting in human, animal or environmental health hazards.
4.1.6 Guaranteed Analysis
Feeds must have an accurate statement of guaranteed analysis. The required guarantees will depend on the intended species and the type of feed. The current Regulations outline these required guarantees in Table 3 of Schedule I and for ingredients within the definition for that specific ingredient. It is proposed that the same approach continue to be taken and we will further examine the requirements for specific feed types. Changes may include, replacing the requirement for a guarantee for Crude Fibre with requirements for Neutral Detergent Fibre (NDF) and Acid Detergent Fibre (ADF) where appropriate (cattle, sheep, horses). Crude fibre is no longer considered the most useful guarantee of fibre in ruminant diets and ADF and NDF guarantees will provide purchasers with a more useful measure. The requirement for a minimum Vitamin C guarantee for fish may be added, and the reference to growth promoting medications may be removed. Additional specific changes may also be considered as further work is done on this.
4.1.7 Lot Number (new)
Under the current Regulations certain feeds must have a lot number. Under the Health of Animals Regulations "every person who manufactures animal food for ruminants, equines, porcines, chickens, turkeys, ducks, geese, ratites or game birds shall keep, for 10 years, records that contain … the lot number and any other information used to identify each lot of animal food". It is proposed that all feeds must have a lot number. This will make the requirement consistent for all feed types. This requirement aligns with the European Union (EU) and Codex requirements. The lot size may be determined by the manufacturer. The lot number must be an alpha-numeric representation, but the specific format and convention may be decided upon by the manufacturer. The presence of a lot number allows for faster, more accurate trace backs in the event of a contamination issue. It also allows the purchaser to contact the manufacturer and request information about a specific lot for which they have questions, thus allowing the manufacturer to provide more information to the purchaser.
4.1.8 Registration Number
Currently, feeds which require registration must have the registration number listed on the label. It is proposed that this requirement continues to be in place. In addition, it is proposed that the registration number must appear in direct relation to the product name and must be clearly legible. This allows inspection staff to verify that the product is appropriately registered and allows purchasers to request information regarding a specific product if required.
4.1.9 Caution, Warning and Other Safety-based Statements
Currently, labels must contain all applicable caution and warning statements. It is proposed that this requirement continues to be in place. Caution and warning statements would include the Ruminant Feed Ban statement for feeds containing prohibited materials, cautions and warnings required by the Compendium of Medicating Ingredients Brochures (CMIB) for medicated feeds, and statements required by ingredient definitions. Labels would also require any additional cautions or warnings which convey useful information to the purchaser. This could include those which are identified in policy, or worker safety information such as suggesting the use of gloves or a dust mask when handling a specific ingredient.
By providing the purchaser with this information they can make an informed purchasing choice and are able to store, use and dispose of the feed product safely. The information indicated by caution and warning statements may include:
- the presence of a specific ingredient or item in the feed which may restrict the species to which the feed can be fed;
- the required withdrawal time to prevent residues which result in a food safety risk; or,
- a maximum feeding rate to help protect animal health.
These statements may also need to be carried forward on to the label of a subsequent product manufactured using the product that has the statement.
4.1.10 Medication Information (Modified)
Under the current regulations, medicated feeds have additional labelling requirements to provide the purchaser with information about the medication in the feed. A similar approach is proposed. It is proposed that medicated feeds will continue to require additional label information relating to the medication. Some flexibility in how the information is provided is being proposed. All feeds containing a medication must identify on the label the name and actual amount of the medicating ingredient in the feed in direct association with the feed name or as part of the guaranteed analysis. They shall also include the appropriate claim or claims for the medicating ingredient(s) used as set out in the CMIB. Feeds containing medications must clearly indicate that they contain a medication. The additional requirement that, if the name and amount of the medication is not directly in association with the feed name (i.e. instead is listed in the guarantees), the term "medicated" must be used as part of the feed name. This approach is consistent internationally and builds upon the previous requirements. Additional flexibility is proposed in terms of where and how the label should indicate that the feed is medicated.
4.1.11 Net Amount (Modified)
Currently, all feeds must indicate a net amount or net weight. This must be expressed in metric measure as per the Weights and Measures Act. It is proposed that this continues to be a requirement. Solid products should be expressed in units of mass and liquid products in units of mass or volume. To provide greater flexibility, weights may be expressed in imperial measure in addition to the required metric measure, but it must be clear which units of measure are used. Imperial units of measure are not required on labels; they are permitted to be used in addition to the required metric units for net amounts. When the net quantity is shown in both metric units and Imperial units, the metric units should be declared first and the two must be grouped together on the label with no intervening information. For example, 20 kg (44 lb).
Have your say:
The CFIA is seeking comments on the proposed mandatory labelling requirements.
4.2 Additional Label Requirements, if applicable
4.2.1 Size/Location of Label Information
As with the current regulations, it is proposed that any information required to be shown on the label of a feed shall be printed conspicuously, legibly and indelibly. To better clarify this requirement, required or mandatory label information shall appear on the main panel of the package label. In cases when a bill of lading also serves as a label the required information must be clear and easily located. Directions for use on a label may refer to a package insert which is included with the package for more detailed directions for use.
4.2.2 Transfer of Cautions, Warnings and Other Statements Forward (new)
It is proposed that when a feed is manufactured using an ingredient or mixed feed which has a caution or warning statement on its label, or for which there is a maximum inclusion rate of an ingredient, this relevant information must be transferred onto the label for the new feed product. For example, if a premix containing a medication with a withdrawal time was used to make a complete feed, this withdrawal time would need to be transferred onto the complete feed. There is currently no requirement for the carry forward of these statements on feeds, with the exception that it may be required for some feeds which are not exempt from registration.
For an ingredient that, due to safety concerns, has a maximum inclusion rate in feeds, this maximum would need to be indicated on the label of the feed in which it was used as an ingredient. For example, ethoxyquin may not exceed 0.015% of the total diet. Any subsequent feed containing ethoxyquin would need to respect this maximum and would need to either indicate the level of ethoxyquin in the product or contain a warning statement that indicates a maximum use of the feed due to the ethoxyquin levels.
4.2.3 Language (bilingual)
Currently feeds must be labelled in French, English or both official languages. However, under the Official Languages Act there is a requirement for health and safety information to be available in both official languages.
From Section 26 of the Official Languages Act:
26. Every federal institution that regulates persons or organizations with respect to any of their activities that relate to the health, safety or security of members of the public has the duty to ensure, through its regulation of those persons or organizations, wherever it is reasonable to do so in the circumstances, that members of the public can communicate with and obtain available services from those persons or organizations in relation to those activities in both official languages.
In order to meet this requirement, it is proposed that any label information which impacts health and safety will be required to be present in both French and English. This could include guarantees, medication information, cautions and warnings, and directions for use.
4.2.4 Multi-lingual Labels
The current Regulations are silent on the use of languages other than French and English on feed labels. Given the realities of global trade and feedback we have received from regulated parties, it is proposed that the modernized regulatory framework provide scope for the multi-lingual labelling of feed and feed ingredients.
Multi-lingual labels would be permitted, however, they must meet Canadian labelling requirements. The label must provide the required Canadian label information in one or both of Canada's official languages and any additional label information must be in compliance with the Canadian regulations. Label information may also be presented in languages other than French or English provided the mandatory Canadian label information is present in all languages. Any additional or voluntary information on the label in other languages must be in compliance with the Canadian regulations and may not be misleading.
If there is misleading, false or conflicting information that may result in the misuse of the product or which poses a safety concern, this information would not be permitted and would be required to be removed from that label. Health and safety risks may vary by country as each country sets their risks based on the consumption patterns in their particular country. The English and/or French information must be printed in the same or larger font than the foreign languages and must be on the main display panel.
4.2.5 Units of Measure (new)
Under the current Regulations, units of measure on a label are required to be in accordance with the Weights and Measures Act. To provide additional flexibility, especially for imported products, it is proposed all units of measure on feed labels must be indicated in metric units, and may also be indicated in other units on a voluntary basis. Solid products should be expressed in units of mass and liquid products in units of mass or volume. It must be clear which units of measure are used. When the weights are shown in both metric units and Imperial units, the metric units should be declared first and the two must be grouped together on the label with no intervening material.
Have your say:
The CFIA is seeking comments on the proposed additional labelling requirements.
4.3 Specific Requirements for Feed Types
Under the current Regulations there are some types of feeds which have specific requirements. These requirements are designed to:
- facilitate exemption from registration;
- address the unique needs of the feed; and
- require labelling information that is relevant to that feed type.
The following is proposed for these feed types:
4.3.1 Single Ingredient Feeds
As per the current requirements it is proposed that labels for single ingredient feeds shall contain all of the guarantees and additional labelling statements that are outlined in the ingredient definition in addition to the standard labelling requirements.
4.3.2 Medicated Feeds
As per the mandatory label information discussed above and the current regulations, it is proposed that medicated feeds will need to indicate the name and amount of the medicating ingredient, any cautions or warnings and their directions for use. This information should reflect the directions for use indicated in each ingredient's respective MIB.
4.3.3 Veterinary Biologics in Feeds (new)
Under the current regulations there is no provision for the inclusion of a veterinary biologic in a feed. It is proposed that feeds may contain a veterinary biologic approved for use in livestock feeds. As with medicating ingredients, the name and amount of the veterinary biologic, the approved claims, intended species, directions for use, and any appropriate cautions or warnings must be indicated on the label. It is proposed to incorporate labelling and composition standards for livestock feeds containing veterinary biologics similar to the way medications are regulated in livestock feeds.
4.3.4 Veterinary Prescription Feeds
As with the current framework, veterinary prescription feeds shall continue to require, in addition to the standard label requirements, the following additional label information:
- the name of the person for whom the feed was manufactured;
- the name of the veterinarian who issued the prescription;
- the name of the feed including the name and amount of the medicating ingredient;
- directions for use including the duration of feeding as per the prescription; and
- any cautions or warning indicated on the prescription including any withdrawal times.
This information must all appear on the main panel of the label in conjunction with the standard required label information.
4.3.5 Imported Feeds
Mixed feeds which are imported into Canada currently require pre-market registration as a measure to verify that they are in compliance with the Canadian regulations. Because foreign facilities are not inspected by the CFIA, pre-market registration allows greater oversight of these feeds. Imported feeds currently have the same labelling requirements as domestically manufactured feeds. The CFIA is exploring options to exempt imported feeds from pre-market registration. One of the conditions of exemption may be additional labelling requirements. Imported feeds would also be required to meet all of the domestic labelling requirements. This will be further explored in a future proposal.
Have your say:
The CFIA is seeking comments on maintaining the status quo for labelling requirements for specific feed types and the proposed addition to allow veterinary biologics to be allowed for use in feeds.
4.4 Prohibitions
There are a number of items which are currently not allowed on feed labels. It is proposed that there continue to be certain prohibitions associated with labelling. These include:
4.4.1 Misleading Information
Labels must be truthful and not misleading. The brand or product name should not deceive or mislead the purchaser with regards to the composition, usefulness or purpose of the product. Feeds should not be unsuitable for the purpose for which they are sold or represented. Feeds should not contain guarantees which are not useful to the purchaser. Guarantees must accurately reflect the contents of the feed. Labels should not contain statements or other information which is not truthful.
Labels shall not contain any variation in size, colour, or placement of the printing that it obscures or emphasizes any part of the information required to be shown on the label. Labels shall not contain misleading or incorrect information or marks.
4.4.2 Government Stamps
No one may reproduce a government stamp on a label without written permission from the Minister. Government stamps may be used to indicate the approval or acceptability of a label; however the stamp is not part of the label and should not be reproduced and used in the market place.
4.4.3 Claims
Under the current Regulations, all claims on labels require pre-market assessment and verification. It is proposed that the basic approach for claims should be that labels should not contain claims other than those which are truthful and verifiable. However, as it is recognized that additional flexibility is required it is also proposed that not all products with label claims will require premarket assessment. In the proposal on ingredient assessment, a scheme for label claims is proposed. This includes the development of a list of permissible claims which would not trigger a requirement for registration. As well, a mechanism for adding additional claims to the list is proposed. Please see the proposal "Feed Ingredient Assessment and Authorization" for more details on label claims.
Have your say:
The CFIA is seeking comments on the proposed prohibitions.
4.5 Voluntary Labelling
In addition to information that is mandatory on the label, information that is required in specific circumstances and general requirements regarding what is and is not allowed on labels, there is additional scope for voluntary label information. This is information that may be placed on the label if the manufacturer wants to add it, but it is not required. Any voluntary label information will need to be in compliance with the general labelling standards.
4.5.1 Brand Name
As with the current framework, it is proposed that labels may include a brand name on a voluntary basis in addition to the product name. A brand means any distinctive mark or trade name, apart from the name of the feed, applied by the manufacturer or registrant to a feed to distinguish it from another feed. Brand names must not deceive or mislead a purchaser with respect to the feeds usefulness or composition. The brand may not be likely to be confused with a brand already applied to a feed.
4.5.2 Additional Guarantees
Under the current framework, any guarantees that are not required on the label of a feed must be approved by pre-market registration. It is proposed that additional guarantees be allowed on labels provided they are accurate, reflective of the contents of the product and would be considered useful to the purchaser. In addition, all guarantees must be verifiable. If a method for verification of the guarantee in a feed matrix is not publically available the company must supply their method upon request to the CFIA or anyone else who requests it. If the CFIA determines that the company supplied method is not appropriate the company must remove the voluntary guarantee until issues with the methodology can be resolved.
4.5.3 Best Before or Expiry Dates
It is proposed that for feeds which do not require a "best before" or "expiry" date, one may be indicated on the label on a voluntary basis. If a date of manufacture is indicated on the label, then the best before date can be indicated as X days after the date of manufacture. Companies would be responsible for determining an appropriate best before date for their product, and must supply information to support it upon request. In the case where multiple ingredients with different best before dates are used in the manufacture of a mixed feed, the best before date expiring first should be used in determining the best before date of the final product.
4.5.4 Other Voluntary Information
It is also proposed that labels may contain other information on a voluntary basis without requiring pre-market approval of the information; however it must be truthful, not misleading and verifiable. In addition, it must be in compliance with the other labelling standards and requirements. If information on labels is found that does not meet these standards during post-market monitoring it must be removed from the label.
Have your say:
The CFIA is seeking comments on the proposed voluntary labelling requirements.
5 Summary
5.1 Proposal Synopsis
The proposed changes to the labelling requirements for feed are one part of the overall approach to modernizing Canada's Feeds Regulations. Comments and feedback on the proposal, from stakeholders and other interested parties will contribute to the development of regulations that are flexible, effective, and in step with current technologies, feed and livestock production systems and markets. Stakeholders are encouraged to provide written feedback on both the components that they support and those which they are not in agreement with.
5.2 Next Steps
Feedback obtained during this consultation will be combined with feedback from the other three modules. A summary document, which will integrate the information from the four separate modules will be published following the individual consultations. This summary document will outline the overall proposed feed framework and will provide an additional opportunity for stakeholder input and is targeted to be released by the end of 2013. Following the development and publication of the proposed overall framework the CFIA will review its service delivery costs and fees within the Feed Program as part of an Agency-wide User Fee modernization initiative.
Objectives for the User Fee initiative include the promotion of effective and responsive service delivery; establishing a consistent and robust approach to user fee development; and fostering an understanding that user fees may be charged for services, products, rights and privileges provided by the Agency. For more details, see the Cost Recovery Policy and Framework.
The Agency will hold public consultations on user fees related to services provided by the Feed Program. Service recipients will have an opportunity to comment on the detailed user fee proposal once it is made public.
5.3 Contact
Your input and feedback is critically important to the success of the regulatory modernization initiative and we strongly encourage you to provide your input. Written comments can be sent to:
Sergio Tolusso
Canadian Food Inspection Agency
Animal Feed Division
59 Camelot Drive
Ottawa, ON K1A 0Y9
Email: Sergio Tolusso
Fax: 613-773-7565
6 Annexes
6.1 Annex 1 – Mock Label
Click on image for larger view
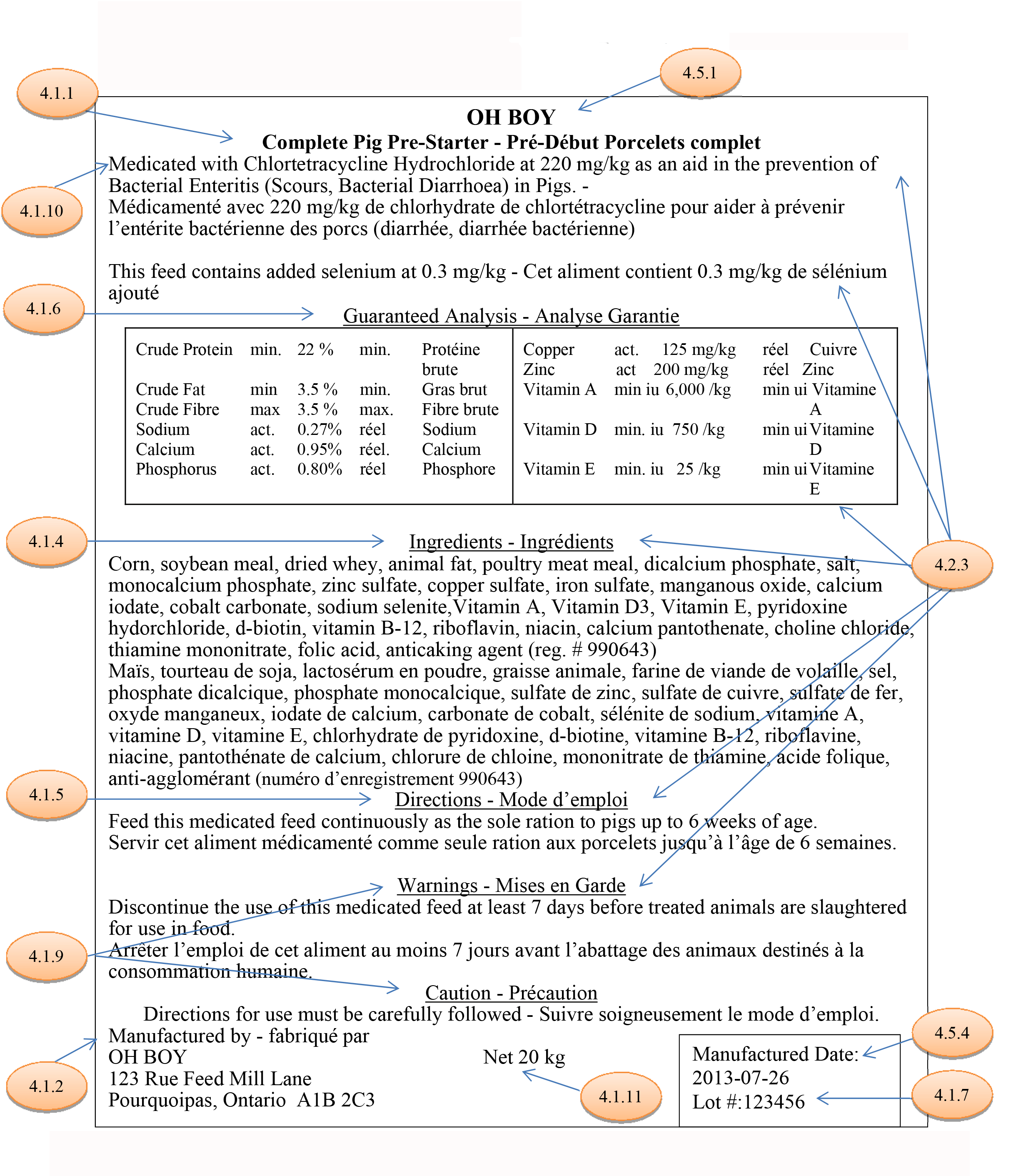
Description for image - Mock Feed Label
This is a mock label that would appear on a package of feed. Highlighted bubbles point to parts of the label. Each bubble makes reference to a section of the Regulatory Framework Proposal.
Section 4.1.1 refers to the requirements for the feed name. There are two components that must be present in the feed name: the purpose or feed type and the species or category of intended livestock.
Section 4.5.1 refers to the brand name. A brand name may be provided voluntarily so long as it does not deceive or mislead a purchaser with respect to the feed's usefulness or composition.
Section 4.1.10 refers to the requirements for medication in a feed. Additional information must be listed on the label. If the name of the medication is not listed, then the term "medicated" must be used as part of the feed name.
Section 4.1.6 refers to guaranteed analysis. These statements must be provided for the guarantees listed in Table 3 of Schedule I.
Section 4.1.4 refers to a new requirement that would require an ingredient list on the label. This is consistent with practices in other international jurisdictions. The use of collective terms would also be allowed to align with labelling practices used in the U.S.
Section 4.2.3 refers to bilingual labelling. Under the Official Languages Act, any label information that impacts health and safety must be present in both English and French. Information that must be bilingual includes guarantees, medication information, cautions and warnings, and directions for use.
Section 4.1.5 refers to the directions for use which must appear on the label.
Section 4.1.9 refers to caution, warning and other safety-based statements which must be on the label. A new product made by combining products with warning statements must show these statements on the new label.
Section 4.1.2 refers to the name and address of the actual place of manufacture. This information must be clearly labelled. Other names and addresses may be voluntarily included.
Section 4.1.11 refers to the net amount or net weight of the product. This information must be on the label shown in metric measure in accordance with the Weights and Measures Act. The equivalent weight in Imperial units may appear directly following the metric so long as the units are grouped together with no intervening information.
Section 4.5.4 refers to other information that may be voluntarily added to a label. The voluntary information must be truthful, not misleading and verifiable. It must also meet the other labelling standards and requirements.
Section 4.1.7 refers to the need to add a lot number to the label. This new feature will speed up trace back searches in the case of contamination issues. It will also further align our labelling practices with other jurisdictions.
- Date modified: