Submission and Review Process for Third Party Submission of Agri-Commodity Biosecurity Standards
This page is part of the Guidance Document Repository (GDR).
Looking for related documents?
Search for related documents in the Guidance Document Repository
1.1 Introduction
The Canadian Food Inspection Agency (CFIA) is responsible, among other things, for certain programs, policies, and initiatives that are focused on protecting Canada's plant and animal resource base. Under the Growing Forward Agricultural Policy Framework, Agriculture and Agri-Food Canada (AAFC) identified the CFIA as the federal government's lead to develop national biosecurity standards and to review standards developed by third parties.
1.2 The Purpose of National Biosecurity Standards
National agri-commodity farm-level biosecurity standards are intended to provide a nationally consistent, systematic, cross-commodity approach to the mitigation of risks posed by disease organisms or pests, and to minimize the risk of zoonoses. These outcomes-based standards are designed taking into account basic biosecurity principles, sound science, and practicality.
The existence and implementation of national biosecurity standards for particular commodities are important in assuring consumers, both domestically and abroad, that Canadian agricultural commodities are produced in a manner that mitigates the risk of disease and pests.
The ultimate outcome of these standards depends on their implementation by the producer, in addition to many factors which may be beyond the direct control of the producer.
1.3 Third Party Submission Process
The Office of Animal Biosecurity (OAB), within the CFIA, works with stakeholders to develop national agri-commodity farm-level biosecurity standards.
For commodities for which the OAB has not undertaken to develop a standard, a third party submission process has been developed. This provides the opportunity for stakeholders engaged in agri-commodity production to develop their own biosecurity standards. Such a standard can then be reviewed to determine whether it meets the criteria of a national agri-commodity biosecurity standard.
1.4 Developing and Writing National Agri-Commodity Biosecurity Standards
National agri-commodity biosecurity standards should be practical, achievable, and effective. They should allow a broad uptake by producers, while accommodating differences in geography, demographics, and production systems across Canada.
Standards should be developed and will be reviewed using the following criteria:
- The standard was developed in broad consultation with government and non-government stakeholders.
- It is supported by a commodity stakeholders' consultative process, with the goal of producer uptake, support, or adoption.
- It incorporates the CFIA's Basic Principles of Biosecurity.
- It is based on known current best practices in both domestic and international biosecurity for the commodity sector.
- It identifies outcomes for a proactive, routine risk management approach in disease and pest prevention and control.
- It is sector specific, and site and regionally adaptable (flexible).
- It is clearly defined and outcome-based (practical, achievable, and effective).
- It is based on the best available knowledge and science.
- It is designed to minimize the risk of introduction and spread of disease(s) and pests that have been identified in a commodity.
- It is responsive and adaptable to emerging diseases and pests and market access requirements.
- It identifies potential linkages and synergies with existing programs and strategies (e.g. traceability, On-Farm Food Safety Program, National Animal Health and Welfare Strategy, animal welfare).
- The final product is available in both official languages (English and French).
2.1 Submission and Review
Commodity groups seeking CFIA (OAB) review of a national agri-commodity biosecurity standard for their sector should be guided by the submission process as indicated in Figure 1.
Click on image for larger view
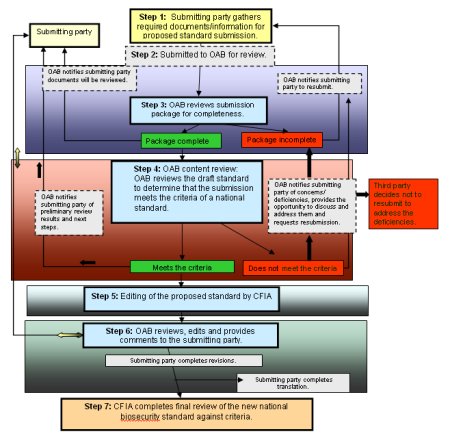
Description for Figure 1: Submission Process for Draft National Biosecurity Standards
Step 1: The submitting party gathers required documents/information for proposed standard submission.
Step 2: The documents and information are submitted to the Office of Animal Biosecurity (OAB) for review.
Step 3: OAB reviews the submission package for completeness.
If the package is complete, the OAB notifies the submitting party that the documents will be reviewed, and proceeds to Step 4.
If the package is not complete, the OAB notifies the submitting party of the concerns/deficiencies to be addressed and recommends they resubmit with the appropriate documentation. The package now starts over with Steps 1-3, or the third party decides not to resubmit to address the deficiencies.
Step 4: The OAB reviews the draft standard to determine that the submission meets the criteria as a national standard.
If the Standard meets the criteria, it proceeds to Step 5.
If the Standard does not meet the criteria, the OAB notifies the submitting party of the concerns/deficiencies, provides the opportunity to discuss and address them and requests resubmission. The package now starts over with Steps 1-3, or the third party decides not to resubmit to address the deficiencies.
Step 5: Editing of the proposed standard by the Canadian Food Inspection Agency (CFIA).
Step 6: The OAB reviews, edit and provides comments to the submitting party. If revisions are required, the submitting party is responsible. The submitting party is also responsible for the translation of the document.
Step 7: The CFIA completes a final review of the national biosecurity standard against the criteria.
2.2 Information Requirements
The following information must accompany the submission:
- Covering letter from the third party submitter that clearly identifies a relationship with and the support of the commodity sector.
- Details of the sponsoring or submitting party, group or organization.
- Contact information for the parties involved in the development of the standard.
- The reason for submission.
- All input (both positive and negative) received in consultation with the following:
- national organizations
- provincial organizations
- federal government departments
- provincial government departments
- other relevant stakeholders
- Letters and other documentation reflecting the view of affected parties on the draft standard.
- The proposed standard.
- Summary and bibliography of references used in development.
Note: Submissions may be made in either official language.
The CFIA reserves the right to validate information provided. Validation may include but is not limited to confirming consultative engagement, confirming support, contacting stakeholders and posting for additional comment.
The information and documents required should be packaged and submitted to the CFIA (OAB) for evaluation. The submission should be mailed to the following address:
Office of Animal Biosecurity
Canadian Food Inspection Agency
59 Camelot Drive
Ottawa, Ontario K1A 0Y9
2.3 Submission and Review Process
The OAB will assess the document package for completeness prior to reviewing the proposed standard. Only complete submission packages will be reviewed. Incomplete submissions will result in the applicant being notified that the package is incomplete and being asked to resubmit.
Submissions that are complete will be reviewed to determine whether the submission meets the criteria for a national agri-commodity biosecurity standard as detailed in section 1.4 above.
2.4 National Biosecurity Standard Format
The following Biosecurity Standard format is provided only as a guide to aid with submission completeness and to illustrate the elements of a National Biosecurity Standard. Applicants are encouraged to create new elements, sub-elements and improvements driven by science, industry sectors, academia and collaboration.
This format includes three main categories (Access Control, Animal Health Management and Operational Management) which contain the basic principles of farm-level animal biosecurity. Below each principle are listed some target outcomes. These have been adapted from the National Avian On-Farm Biosecurity Standard and illustrate only examples of outcome-based targets; desired outcomes will vary with individual production systems.
Example: Animal/Poultry On-Farm Biosecurity Standard
Minimizing disease transmission in animal populations requires managing the exposure of animals and their environs to potentially contaminated people, equipment, animals, and things. Areas occupied by animals or used in their daily care must be protected by access procedures and movement controls to elevate the health status of these areas from the uncontrolled movements and contacts that occur outside the premises. These areas are termed "biosecure zones."
Access Control
1. Designation of Zones – Zones of restricted and controlled access are established on the premises. Access to and from the barns and premises is controlled through the establishment of protective zones and controlled access points.
Examples of Target Outcomes:
- Recognizable zones and access points are in place.
- Visual indicators are in place to define biosecure zones.
2. Entry/Movement/Exit Controls for Designated Zones – Movement into, within and out of the restricted and controlled access zones is governed by standard operating procedures (SOPs).
Examples of Target Outcomes:
- People working on the premises are knowledgeable of and understand the importance of and rationale behind biosecure zones.
- Access to biosecure zones is controlled by appropriate measures and routine procedures. Tools, equipment and facilities necessary to accomplish the established procedures are available, functional and maintained for their required purpose.
Animal Health Management
3. Animal Introduction/Movement/Removal – Animal introductions, movements within the premises and removal from the premises follow the SOP's designed to minimise the probability of spread of disease organisms.
Examples of Target Outcomes:
- Each placement or removal of animals is recorded and carried out with appropriate scheduling, isolation or segregation to minimize the introduction or spread of disease.
- The down time between animal groups is optimized.
- More stringent additional biosecurity measures are implemented at entry points to zones when "all in /all out" scheduling and downtime is not practical.
4. Ongoing Monitoring of Health Status and Response – Workers on the premises have training in the ongoing monitoring of the health status of the animals/birds on the farm including an action plan for a response to an animal health incident.
Examples of Target Outcomes:
- Individuals who monitor animals are knowledgeable and experienced in monitoring animal health, the recognition of disease conditions and timely response protocols.
- Daily procedures for observation and culling if necessary are followed.
- A daily mortality log is maintained.
- Unusual morbidity or mortality triggers contact with a veterinarian and disease diagnosis action. Suspicion of diseases which are contagious, of economic importance or reportable triggers a "disease response plan" that provides guidance to individuals on the appropriate procedures to follow.
Operational Management
5. Mortality and Manure Management – Dead stock and manure are managed to minimise the risk of spread of disease.
Examples of Target Outcomes:
- Daily procedures are followed with respect to mortalities, including collection and removal from production areas.
- A mortality storage system, which protects the carcasses from scavengers and insects until final disposal, is used on the premises.
- Carcass disposal, including any on-farm disposal (incineration, composting and burial), is done in accordance with provincial or municipal guidelines. If a rendering service is used, the pickup is performed to minimize any biosecurity risk.
- Manure is suitably handled and stored to minimize the risk of transferring disease organisms to animals.
6. Premises, Building, Equipment and Vehicle Sanitation – SOP's are in place regarding the routine sanitation and disinfection of the premises and the equipment and vehicles moving onto and off the premises.
Example of a Target Outcome:
- A sanitation program is in place that applies to premises, building, equipment and vehicle sanitation.
7. Facility Maintenance – The facility is maintained in a state to allow the implementation of the SOP's for the biosecurity program.
Example of a Target Outcome:
- A program for facility maintenance is in place.
8. Water/Feed/Bedding Management – Inputs to production (water, feed and bedding) are obtained from sources to minimise possibility of introduction of disease organisms, and handled and stored in a manner to maintain the safety of the inputs.
Examples of Target Outcomes:
- A water management program is in place to ensure that water is potable and meets local guidelines for animal consumption.
- Feed is obtained and stored in a manner that minimizes the risk of contamination by pathogens.
- Bedding is obtained and stored in a manner that minimizes the risk of contamination by pathogens.
9. Pest Control Program – An integrated pest management system is in place.
Examples of Target Outcomes:
- An integrated pest control program is present.
- Garbage is effectively and safely disposed of.
10. Biosecurity Program and Training – An enterprise has a well-defined, written biosecurity program in place including the above elements, and provides training and training materials for workers.
Examples of Target Outcomes:
- All people working on the premises are knowledgeable of, and understand the rationale behind and importance of, biosecurity and biosecurity protocols.
- All people working on the premises have reviewed the applicable biosecurity-related instructions as needed, based on their assigned tasks.
2.5 OAB Draft Biosecurity Editing Process
The OAB will guide the document through a CFIA editing process.
OAB Reviews Edits With Submitter
The document will be reviewed with the submitter to ensure that the latter agrees with the draft.
A national biosecurity standard must be available in both official languages. It is the responsibility of the submitter to have the standard translated. The translated version of the standard will be submitted to the OAB for approval of the translation.
Publication
The final step after final revision is publication of the national biosecurity standard. In an effort to assist industry, the CFIA website may link to the commodity sector site (where the standard is posted) for up to 36 months. After 36 months, the submitter must submit a request for review of the standard if the submitter wishes to have the posting continue. The CFIA reserves the right to remove the link from its website at any time if the standard is not maintained to reflect current biosecurity principles. Amendments to the standard are the responsibility of the submitter and the holder of the commodity sector site. The CFIA must be notified in advance of any amendment to the standard and will evaluate the impact of such amendments when determining whether to continue including the link on the CFIA website. In all cases, the CFIA will notify the applicant if a decision is made to remove the link from the CFIA website.
- Date modified: