Canadian Grain Sampling Program Audit Manual
This page is part of the Guidance Document Repository (GDR).
Looking for related documents?
Search for related documents in the Guidance Document Repository
Several changes have been made to the document, including the following:
- introduction clarification of third party sampler
- 1.1 provided examples of differences in a third party sampler manual
- 1.2 further details on evaluation audits and the process of approval were added
- 1.3 information was provided on how to assess facilities not submitting samples during the year
- 2.0 clarification was provided around non-conformances detected outside of audits
- 2.0 the recommended style for a Corrective Action Request (CAR) number was added
- 3.0 further details were provided around suspension
- 4.0 Additional responsibilities were added for the company and the Regional Program Officer
- 4.4 "Area Grains and Oilseeds Program Specialist" section removed
- 5.0 step 1 Clarification provided around determining the scope of the audit, having a sampler present, and an updated list of documents/tools needed for the audit
- 5.0 step 2 Clarification provided on filling out the audit report
- 5.0 step 3 Further explanation around observations was added
- 5.0 step 3 Company status was defined
- 5.0 step 4 Updated instructions for the closing meeting, completing the audit forms and issuing Corrective Action Requests
- 5.0 step 5 Instructions added for follow-up on Corrective Action Requests
- 5.0 step 6 Addition to send a copy of the completed audit report to the company manager
- appendices revisions completed to the audit report, Audit Detail Report and Corrective Action Request Form, with all forms being converted to fillable PDF format
- various editorial changes have been made to improve the format and clarity of the text.
On this page
- Contact and review
- Endorsement
- Introduction
- Scope
- References
- Definitions, abbreviations and acronyms
- 1.0 Audit and documentation review
- 2.0 Non-conformance
- 3.0 Suspension
- 4.0 Roles and responsibilities
- 5.0 CGSP Audit work instructions
- Appendix 1 – Canadian Grain Sampling Program Audit Report and Canadian Grain Sampling Program Audit Detail Report
- Appendix 2 – CGSP Corrective Action Request
- Appendix 3 – CGSP Notice of Suspension from the Canadian Grain Sampling Program
Contact and review
The contact for this document is the Field Crops and Potato Section of the Canadian Food Inspection Agency (CFIA).
This directive will be updated as required. For further information or clarification, please contact the Canadian Food Inspection Agency (CFIA).
Endorsement
Approved by:
National Manager, Field Crops and Potato Section
Date
Introduction
The Canadian Grain Sampling Program (CGSP) Audit Manual is a supplementary document to CFIA directive D-10-02: The Canadian Grain Sampling Program (CGSP). This audit manual is for use by auditors and outlines the approval and auditing requirements for:
- participating facilities sampling grain for export
- participating third parties sampling grain for export
Facilities or third parties (a company or individual that takes samples of grain or grain products for export on behalf of an exporter and has no financial stake in the processing or shipping of the grain or grain product) seeking approval under the CGSP will be referred to as company(ies) in this document.
Scope
This document outlines the criteria and procedures used to approve and audit companies participating in the CGSP. It also provides guidelines to determine compliance under the CGSP.
References
- International Organization for Standardization (ISO) 9000:2015 Quality management systems – Fundamentals and vocabulary
- International Standard for Phytosanitary Measures (ISPM) No. 5 Glossary of Phytosanitary Terms, FAO (updated annually) - PDF (169 kb)
- D-10-02, The Canadian Grain Sampling Program (CGSP)
Definitions, abbreviations and acronyms
Definitions for terms used in this document can be found in the Plant Health Glossary of Terms.
1.0 Audit and documentation review
1.1 CGSP Audits
A quality management system is a method of operation that incorporates an organizational structure, procedures, processes (for example, quality management system procedure, quality management system manual(s), quality control protocols, audit procedures, etc.) and resources, needed to implement quality management. A quality management system will be utilized to ensure samples taken for phytosanitary purposes meet criteria established by the CFIA. The company must document the procedures that they will be following in their quality management system to meet the conditions of the CGSP. This documentation is the company's quality management system manual, (herein referred to as the manual). Third party samplers will have additional details they must capture in their manual that differ from a facility sampler (for example, where samples may be taken, the types of sampling equipment that may be used, whether they will be using their own sampling equipment or equipment owned by the facility.
Audits will be composed of a documentation review and a practical component. The document review will be conducted to verify that the company meets the requirements prescribed in D-10-02 and is operating within the limits of the instructions of their manual. The practical component will be conducted on location, where sampling takes place.
1.2 Evaluation audit
Before allowing a company to participate in the CGSP, the CFIA auditor will conduct an evaluation audit. An evaluation audit is a systematic examination to verify the ability of the company to fulfill the requirements of the CGSP as outlined in their manual. Companies successfully completing an evaluation audit will get a copy of the approved application with a unique CGSP identification number (Appendix 1; D-10-02). If the evaluation audit has identified deficiencies, the manager of the company will be provided with detailed information in writing on what deficiencies need to be addressed prior to approval. Use the audit report in Appendix 1 of R-006 and record deficiencies on the Audit Detail Report. Additional evaluation audits may be required to verify the correction of deficiencies identified in the initial evaluation audit. If the evaluation audit determines that the company can be approved to participate in the CGSP, the company will be provided with the completed and signed application form containing the company's CGSP number (a letter of approval is optional).
Not all aspects of the audit checklist may be able to be assessed fully at the time of the evaluation audit, but the company must still be able to demonstrate that they are familiar with the requirements (that is, walk the auditor through the entire process that will be followed).
1.3 System audits
The system audit is a review by the CFIA of the organizational structure, procedures, processes and resources used by participating companies in implementing the CGSP. A system audit will be performed by the CFIA at a minimum of once a year but the frequency can be increased at the discretion of the CFIA if any compliance issues arise.
The system audit will involve assessments of how the company meets the requirements of the CGSP, including review of the company's manual and records. Additional audits may take place as a follow up to corrective actions taken by the company as required by the CFIA. The timing of the system audits will be based on the activity of the company throughout the year. The CFIA will provide a CGSP Audit Report (Appendix 1) as part of the system audit.
In cases where a facility may not submit samples during the year, but wants to remain on the CGSP, not all aspects of the audit checklist may be able to be assessed fully at the time of the system audit. However, the company must still be able to demonstrate that they are familiar with the requirements (that is, walk the auditor through the entire process that will be followed).
2.0 Non-conformance
Activities or products that are found to be in contravention with the CGSP are considered non-conformances. Non-conformances can be detected during the internal audits conducted by the company, during an audit conducted by the CFIA, or outside of CFIA/company audits (for example, issues with samples or sampling process). A CGSP Corrective Action Request (CAR) will be issued by the CFIA for each non-conformance that is detected during the audit (Appendix 2). Non-conformances identified outside of an audit can be noted to be assessed during the next audit if minor, or dealt with immediately if major or critical (automatic suspension). Company may need to make changes to their manual as part of their response to a CAR. Observations on suggested changes or improvements to a company's manual or procedures will be documented on the CGSP Audit Detail Report (Appendix 1).
All CARs reported must be classified as either being critical, major, or minor in nature. The number and class of non-conformances found will determine the status of the company and the subsequent auditing frequency. Each CAR is numbered consecutively per company and per audit. The recommended format for a CAR number is the date the audit started followed by a consecutive series of numbers for the CARs. For example, 2021-10-12-01, 2021-10-12-02 and so on. Classification of non-conformances will be based on an evaluation of the associated risk and whether the integrity of the CGSP has been compromised.
Each CAR must be addressed with a proposed action plan from the company which must include a detailed description of the measures that will be implemented to prevent recurrences of the non-conformances and a time frame for completing the corrective actions. The action plan must be approved and its implementation verified by the auditor. Failure of the company to implement the approved action plan may result in suspension from the CGSP.
3.0 Suspension
Prior to suspension of a company from the CGSP, the auditor should consult with the Regional Program Officer and their Supervisor. If the company is suspended, they will receive a CGSP Notice of Suspension from the Canadian Grain Sampling Program (Appendix 3) and their name will be removed from the CGSP Participant list.
A company that has been suspended may re-apply for certification under the CGSP at any time, provided a detailed report of the corrective actions taken to address the previous non-conformances is included with the application form (D-10-02 Appendix 1). The CFIA will conduct an evaluation audit to verify if the corrective actions are adequate and review their manual. If approved, the CFIA will re-list the company on the internal CGSP participant list.
Suspended companies cannot submit sample(s) for phytosanitary certification to the CFIA or through the Recognition of Export Grain Analysis by Authorized Laboratories (REGAL) program. The only option available for export sampling will be product sampling by the CFIA or by a CGSP third party sampler.
4.0 Roles and responsibilities
4.1 Company
The responsibilities of the company are to:
- maintain written procedures and records in compliance with requirements of the CGSP
- assist the CFIA with audit activities including provision of copies of written procedures and records for review as requested
- answer auditor's inquiries related to the implementation of written programs and other procedures used in the company
- identify and correct non-conformances in a timely and appropriate manner
- provide organizational chart showing function and responsibilities of staff members involved in CGSP (optional)
- notify the CFIA of changes to the manual
- notify the CFIA of major/critical internal CARs (during or outside internal audits)
4.2 Auditor
The responsibilities of the auditor are to:
- ensure the program requirements of the CGSP are being met by participants
- ensure all documents used in the conduct of audit activities (including audit procedures) are current by using the latest versions available on the CFIA website
- conduct audit in accordance with the national frequency
- ensure the condition of all tools and safety equipment is suitable for their intended use (for example, clean, functioning)
- seek guidance and clarification of program and audit through appropriate channels as required
- advise Supervisor/Regional Program Officer of problems or concerns regarding audit.
- communicate audit results to the company/operator in a professional manner using the correct documentation
- assess the company's corrective action submitted in response to CAR
- follow-up on company's corrective action plan implementation in response to CAR
- ensure that complete record of audit is available (some records exist only electronically, others are available as hard copy)
- maintain all audit notes
4.3 CFIA Regional Program Officer
The responsibilities of the Regional Program Officer (RPO) are to:
- provide clarifications to audit staff in relation to program or policy
- seek advice on questions related to the audit process, program or policy issues from the CFIA Operational Guidance and Expertise Plant Health - Fields Crops and Inputs section via the internal Request for Action (RAF) process
- provide support to audit staff in relation to non-compliance or enforcement issues
- as required, conduct evaluation audits and add companies to the CGSP Participant list
- as required, verify recommendations from the field for the suspension of a company
- revise CGSP Participant list when a company is suspended/becomes inactive
5.0 CGSP audit work instructions
The company audit process is divided into 6 separate steps, see diagram below. Each of the 5 steps is divided into detailed procedures. The Canadian Grain Sampling Program Audit manual provides direction to the auditor on how to deliver each individual step of the audit process.
The following list outlines the stages of the CGSP audit:
- step 1 – Preparation for the audit
- step 2 – Gathering of Information
- step 3 – Determining the level of compliance
- step 4 – Communication of the audit results
- step 5 – Follow-up audit
- step 6 – Closing the audit and documentation
Step 1 – Preparation for the audit
Initiate audit (auditor)
- Review annual audit work plan for CGSP companies (CGSP company audit should be a minimum of once a year)
- Identify company requiring audit
- Determine audit team and ensure staff have required competencies and designation (that is CFIA understanding audit course or equivalent).
Determine the scope of the audit
- Review company file including the manual and results of previous audits
- Review issues or actions since the last audit
- Review samples submitted and choose specific samples to have the facility trace back to ensure all paperwork is available (if company has been active)
- All possible audit elements are not listed in the audit detail report
- The elements that are listed are those that should be checked during any audit
- The auditor can and should add other elements to match the situation that they are seeing at the facility or with the third party sampler
Set up an appointment with the company
- Contact company manager to arrange date and time for audit
- Determine safety equipment requirements
- Confirm audit date, time and scope with the company by letter, fax or email
- Request to have someone that typically performs sampling tasks be available to demonstrate sampling (this includes sample submission tasks)
Review current regulatory and reference documents
- Review any relevant reference documents available on the CFIA website including CGSP directive and CGSP audit manual
Gather required audit documents, tools and protective equipment
- Audit documents
- The Canadian Grain Sampling Program Audit Manual
- Blank digital/paper copy of CGSP Audit Report (Appendix 1)
- Plant Protection Act and regulations
- D-10-02: The Canadian Grain Sampling Program
- Blank digital/paper copies of CGSP Corrective Action Request (CAR) (Appendix 2)
- Safety equipment (as required by company)
- Safety boots
- Hard hat
- Gloves
- Dust mask
- Ear plugs
- Safety glasses/goggles
Additional equipment may be required depending on the company's safety policy
- Miscellaneous supplies and tools
- Flashlight (class II, group G, division 1, approved)
- Pen, pencils
- Tablet/laptop
- Smartphone (use for camera and calculator)
Step 2 – Gathering of information
Conduct opening meeting
- Introduce all audit staff and explain roles
- Define objective and the scope of the audit
- Confirm details of the company's operation
- Outline audit schedule and procedures
- Discuss changes in program design and/or regulations
- Review any outstanding non-conformances
- Explain process for Corrective Action Request (CAR)
- Identify company staff and resources required
- Confirm company contact – official communications link
- Confirm safety requirements
- Discuss date and time for the closing meeting
- Keep complete and accurate notes of all items discussed
Gather audit information
- Collect facts through audit observations, staff interviews and document reviews (procedures and records)
- Confirm company's records and procedures by utilizing a second audit method: cross-audit through company staff interviews and/or observations (this will allow the auditor to cross-reference the information gathered for an item)
Review the company's procedures
- Assess procedures based on the CGSP and their manual
- Audit comments on the CGSP Audit Report (Appendix 1) must contain:
- information which clearly identifies specific procedures assessed (how, where, when, etc.)
- name and title/area of responsibility of company staff interviewed
- description of any non-conformance observed
Review the company's records
- Select and assess records based on the company's manual
- Audit comments must contain:
- information which clearly identifies the specific records assessed
- name and date of record
- number of records
- name, title and area of responsibility of the company staff interviewed
- description of any non-conformance observed
Interview company staff
- Interview company staff as necessary to confirm company procedures and records
- Conduct interviews using effective interview techniques
- Audit comments must contain:
- name, title and area of responsibility of company staff interviewed
- information which clearly identifies specific procedures and/or records verified per above
- description of any non-conformance observed
Observe conditions in the company and practices employed
- Observe company staff at work
- Audit comments must contain:
- area of the company/procedure observed
- name, title and area of responsibility of the personnel observed
- information which clearly identifies specific procedures and/or records verified
- description of any non-conformance observed
Record audit comments on CGSP Audit Report
- At the time of audit, record audit comments as outlined above, on the CGSP Audit Report (Appendix 1)
- Develop audit comments based on information gathered at the time of audit
- Keep any rough notes in the audit file
- Ensure recorded audit comments are clear, concise, accurate, complete and contain sufficient detail to support conformance level
- If inspection findings show that the facility is applying procedures as noted in their manual, it would suffice to note "as per manual" as opposed to documenting all the specific details
- Where inspection findings show possible non-conformance, more detailed notes are required
- Provide any additional comments in the "Comments" section
- For third party audits, provide the contact details for the facility visited during the audit
Identification of non-conformance that requires immediate control actions
- If during the course of an audit, the auditor encounters non-conformance that may seriously impact on phytosanitary certification, the CFIA must immediately initiate actions to control the affected product and inform the company
- Communicate non-conformance to the CFIA supervisor/RPO to inform them of the situation
- Complete the task and assign a compliance level supported by clear, concise, accurate and complete audit comments
- A CGSP Corrective Action Request (CAR) (Appendix 2) is issued if a corrective action is required before the closing meeting
- If critical, the company should be suspended and a CGSP Notice of Suspension from the Canadian Grain Sampling Program (Appendix 3) should be issued
Step 3 – Determining level of compliance
Critical non-conformance
- Audit findings that indicate the integrity of the CGSP is in jeopardy are considered to be critical non-conformances
- The company is immediately suspended from the CGSP if any critical non-conformances are found
- For suspended companies, the only option available for export sampling will be product sampling by CFIA or CGSP third party sampler
Major non-conformance
- Major non-conformances are isolated incidents of non-conformance, which do not immediately impact the integrity of the certified product but may become a problem over time
- Corrective actions must be carried out to the satisfaction of the CFIA within 10 business days of receiving the CAR. The corrective actions will generally require a change to the manual and will include measures to prevent a reoccurrence
- If 2 or more major non-conformances are detected during an audit, or if the company fails to carry out the required corrective actions within the specified time period, the non-conformances will be assessed as critical and the company will be immediately suspended from the CGSP
Minor non-conformance
- Minor non-conformances are those that do not immediately or significantly affect the status of the product, but could lead to a major non-conformance if not addressed
- Corrective actions must be undertaken by the company within a time limit specified by the CFIA
- If 3 minor non-conformances are detected in any one audit this is considered equivalent to 1 major non-conformance (for example, 4 minor non-conformances are equal to 1 major non-conformance and 1 minor non-conformance. Similarly, 6 minor non-conformances are equal to 2 major non-conformances, which constitutes a critical and the company would be immediately suspended from the CGSP)
Observations
- Observations are points or practices, which could be used to improve the company's quality management system
- An observation may be used to identify a situation of concern that does not warrant a CAR, or to highlight, suggest or reinforce particular practices (for example, procedures may be compliant based on the CGSP, but it is not what is in their manual)
- Observations should be recorded on the audit report in the Comments section (Appendix 1) and consecutively numbered and recorded on the Audit Detail Report
Company status
- "Approved" refers to a successful evaluation audit
- "Standard" refers to a successful system audit
- "Under Review" refers to an unsuccessful evaluation audit or an unsuccessful system audit that may lead to a suspension
- Note: if this report is being delivered after the decision to suspend the facility or company then the term "Under Review" can be changed to "Suspended". A Notice of Suspension letter will also need to be issued
Step 4 – Communication of audit results
Closing meeting
- Conduct the closing meeting with the company management and discuss the findings from the inspection
- If additional clarification is needed to categorize non-conformance or to review the information, notify the regulated party that you will contact them to provide further information on the results of the inspection
- The onsite closing meeting may be informal as the audit findings may not be formalized until a later date
Complete the audit forms
- Complete the audit forms and send a copy to the company manager (Appendix 1).
Issue Corrective Action Requests
- Complete Appendix 2 for any non-conformances and provide them to the company management
- Calculations on multiple major/minor non-conformances being elevated to a higher level of non-conformance can be indicated here
- Note the dates for follow-up, including dates for provision of Corrective Action Plan (CAP) as well as the dates for implementation of the corrective actions
- Save audit form and supporting documents
Step 5 – Follow-up audit (if required)
Prepare to conduct follow-up audit to reassess identified non-conformances
The following activities must be completed prior to carrying out the follow-up audit:
- If required, follow-up on Corrective Action Requests. This may require a follow-up audit or a request to provide documentation showing the non-conformance has been resolved
- The following activities must be completed prior to carrying out the follow-up audit:
- review the details of the non-conformance from the last audit conducted at this company
- review any CAPs provided
- contact the manager of the company in advance to arrange the date and time for the follow-up audit
- confirm safety equipment requirements
- assemble the audit and safety equipment as required to conduct the follow-up audit
- conduct a follow-up audit within a maximum of 10 calendar days after the corrective actions were to be implemented
Conduct follow-up audit to reassess identified non-conformances
- Go onsite and confirm the following:
- proposed corrective measure has been implemented within the agreed time frames
- areas of non-conformance identified during last audit have been fully addressed; and
- company has implemented corrective measures that effectively prevent recurrence of the non-conformance
Follow-up audit – Unacceptable implementation of corrective action
- If during the follow-up audit, it is observed that the implementation of the Corrective Action has not corrected the non-conformance, add additional comments in the appropriate box on the CAR that corrective actions taken are not satisfactory
- It is important to date and initial each added comments on both document
- If the company fails to carry out the required corrective actions for major non- conformance within the specified time period, the non-conformances will be assessed as critical and the company will be immediately suspended from the CGSP
- If the company fails to carry out the required corrections for minor non-conformances within the specified time period, the non-conformances will be assessed as major non-conformances
- Additional follow-up audit may be required
Follow-up audit – Acceptable implementation of corrective actions
- If during the follow-up audit, it is observed that the implementation of the corrective action has corrected the non-conformance, enter comments in the appropriate box for which the non-conformance was noted
- This must be linked with the audit report from the initial audit
- Close, sign, date the CAR and provide copy of the CAR form to company, indicating that the corrective action taken by the company is acceptable
Step 6 – Closing the audit and documentation
Complete audit file
This file or part of this file can exist as electronic or hard copy. When the company audit has been concluded, the file is reviewed to ensure it contains all applicable documents required to support the audit, including:
- confirmation of audit (letter, fax, email)
- audit rough notes (includes audit form used on-site)
- copies of records related to non-conformance
- other records copied for review to be kept in file until the next audit at that company
- closing meeting notes
- completed CGSP Audit Report (Appendix 1)
- completed CGSP Corrective Action Request (CAR) (Appendix 2)
- CGSP Notice of Suspension from the Canadian Grain Sampling Program (Appendix 3) if applicable
Appendix 1 – Canadian Grain Sampling Program Audit Report and Canadian Grain Sampling Program Audit Detail Report
The Canadian Grain Sampling Program Audit Report
Click on image for larger view

Description for form: CGSP Audit Report
Canadian Grain Sampling Program, report number, evaluation or system
Company name, address phone number
CGSP identification number, date of audit, company manager, CFIA auditor(s)
Other company representatives present at the time of the audit:
Audit of scope and objectives
The objectives of this audit is to ensure compliance with the requirements of the D-10-02, The Canadian Grain Sampling Program. The scope is to verify that company staff, procedures and records are in place to ensure that samples taken by the company meet the requirements to be used as the basis for issuance of Phytosanitary Certificates by the CFIA.
For an evaluation audit: See attached audit detail report for deficiencies to be addressed before approval is granted.
For a system audit: Any observations or non-conformities will be documented and rated depending on the impact they have on the integrity of the program. Critical, Major and Minor non-conformities are summarized below and documented on the attached Corrective Action Requests. Observations will be numbered and documented in the audit detail report. Unresolved observations may affect a company's status at subsequent audits.
Reference documents
D-10-02, The Canadian Grain Sampling Program, date
R006, Canadian Grain Sampling Program audit manual, date
Canadian Grain Sampling manual for: , date
Last audit system report: number: , date
Audit Detail Report see page of the attached report.
Summary of system audit findings
Type, description (use tables/inserts rows to add rows), Corrective Action Request number
- Critical
- Major
- Minor
Comments
Company status, approved, standard, under review
CFIA auditor, signature, date
Distribution of audit report
Cc Other auditors, regional program officer
Version date June 2021
The Canadian Grain Sampling Program Audit Detail Report
Click on image for larger view
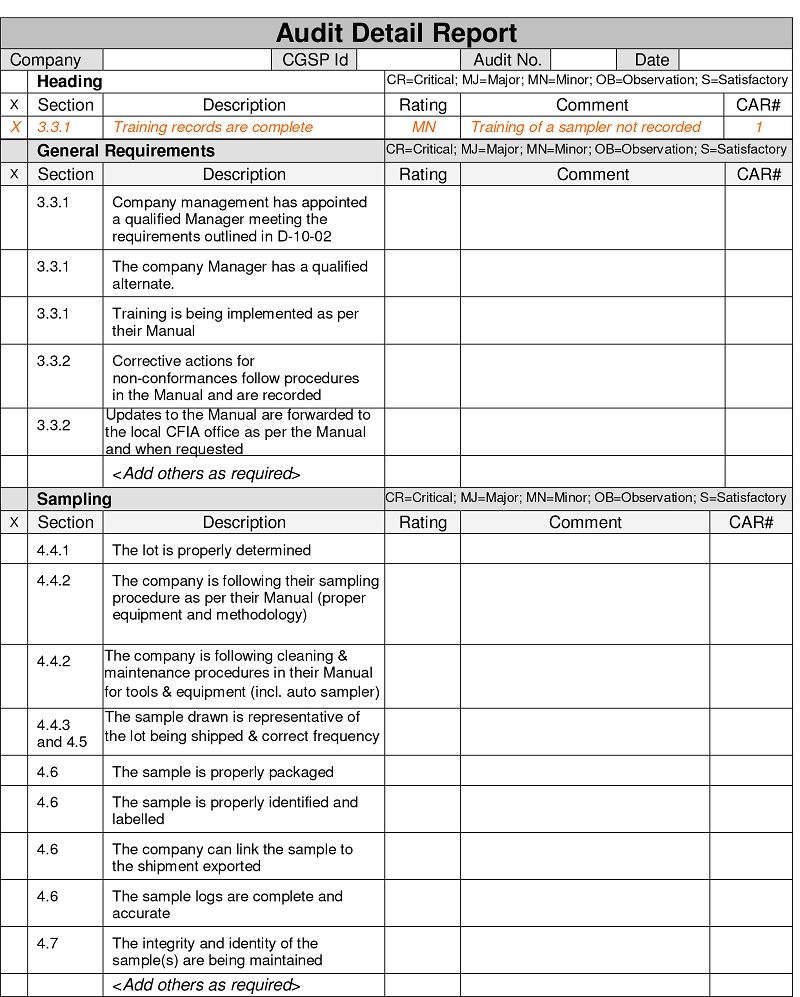
Description for form: Canadian Grain Sampling Program Audit Detail Report
Company
CGSP identification number
Audit number
Date
Heading
CR=critical; MJ=major; MN=minor; OB=observation; S= satisfactory
This table has 5 main sections each comprised of 5 columns. The headings for the 5 columns are:
- section
- description
- rating
- comment
- Corrective Action Request (CAR) number
An example is provided on how to fill the report.
Section: 3.3.1
Description: Training records are complete
Rating: MN
Comment: training of a sampler not recorded
CAR number: 1
General requirements
Section: 3.3.1
Description: Company management has appointed a qualified Manager meeting the requirements outlined in D-10-02
Rating:
Comments:
CAR number:
Section: 3.3.1
Description: The company Manager has a qualified alternate
Rating:
Comments:
CAR number:
Section: 3.3.1
Description: Training is being implemented as per their manual
Rating:
Comments:
CAR number:
Section: 3.3.2
Description: Corrective actions for non-conformances follow procedures in the manual and are recorded
Rating:
Comments:
CAR number:
Section: 3.3.2
Description: Updates to the manual are forwarded to the local CFIA office as per the manual and when requested
Rating:
Comments:
CAR number:
Add others as required
Sampling
Section: 4.4.1
Description: The lot is properly determined
Rating:
Comments:
CAR number:
Section: 4.4.2
Description: The company is following their sampling procedure as per their manual (proper equipment and methodology)
Rating:
Comments:
CAR number:
Section: 4.4.2
Description: The company is following cleaning and maintenance procedures in their manual for tools and equipment (including auto sampler)
Rating:
Comments:
CAR number:
Section: 4.4.3 and 4.5
Description: The sample drawn is representative of the lot being shipped and correct frequency
Rating:
Comments:
CAR number:
Section: 4.6
Description: The sample is properly packaged
Rating:
Comments:
CAR number:
Section: 4.6
Description: The sample is properly identified and labelled
Rating:
Comments:
CAR number:
Section: 4.6
Description: The company can link the sample to the shipment exported
Rating:
Comments:
CAR number:
Section: 4.6
Description: The sample logs are complete and accurate
Rating:
Comments:
CAR number:
Section: 4.7
Description: The integrity and identity of the sample(s) are being maintained
Rating:
Comments:
CAR number:
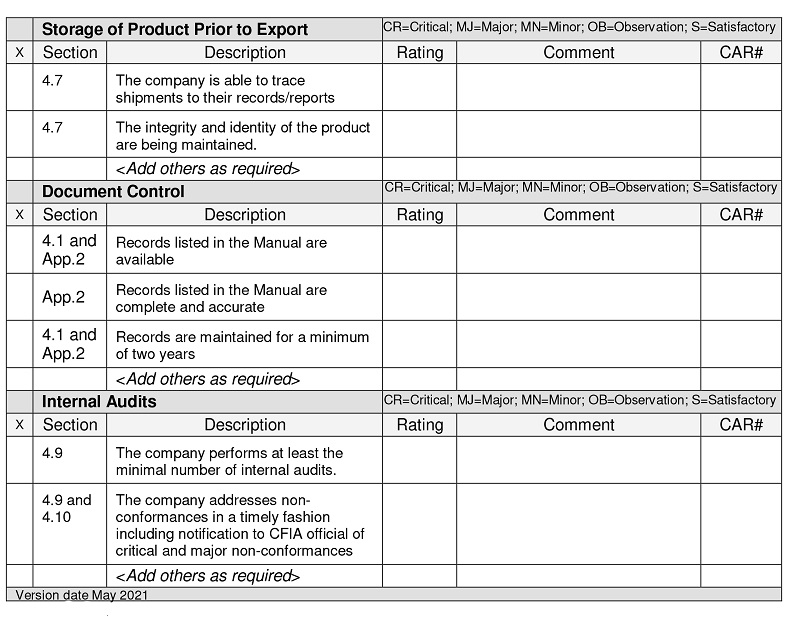
Storage of product prior to export
Section: 4.7
Description: The company is able to trace shipments to their records/reports
Rating:
Comments:
CAR number:
Section: 4.7
Description: The integrity and identity of the product are being maintained
Rating:
Comments:
CAR number:
Add others as required
Document control
Section 4.1 and appendix 2:
Description: Records listed in the manual are available
Rating:
Comments:
CAR number:
Section Appendix 2:
Description: Records listed in the manual are complete and accurate
Rating:
Comments:
CAR number:
Section: 4.1 and appendix 2
Description: Records are maintained for a minimum of 2 years
Rating:
Comments:
CAR number:
Add others as required
Internal audits
Section: 4.9
Description: The company performs at least the minimal number of internal audits
Rating:
Comments:
CAR number:
Section: 4.9 and 4.10
Description: The company addresses non-conformances in a timely fashion including notification to CFIA official of critical and major non-conformances
Rating:
Comments:
CAR number:
Add others as required
Version date May 2021
Appendix 2 – Canadian Grain Sampling Program Corrective Action Request (CAR) Form
Click on image for larger view
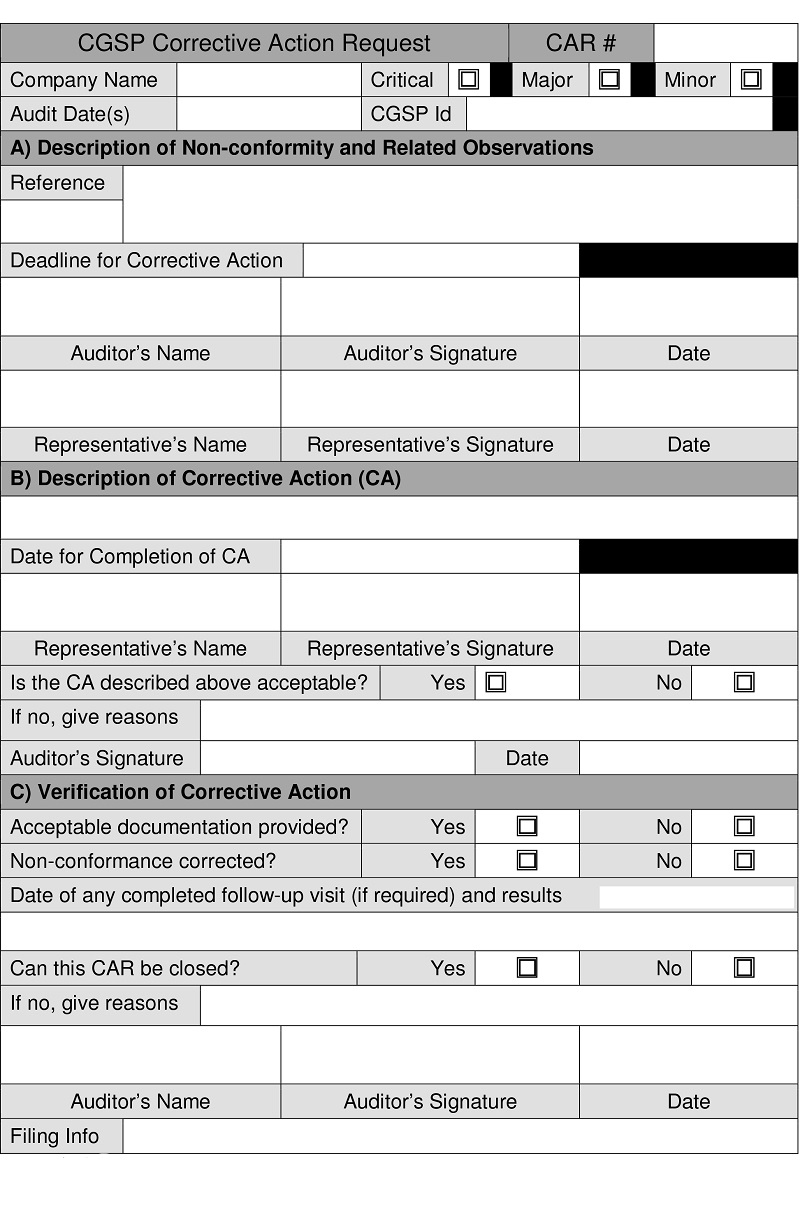
Description for form: Canadian Grain sampling Program Corrective Action Request
Canadian Grain Sampling Prog4ram Corrective Action Request
Corrective Action Request number
Company name
Critical, major, minor
Audit date(s)
Canadian Grain sampling Program identification number
A) Description of non-conformity and related observations
Reference
Deadline for corrective action
Auditor's name, auditor's signature, date
Representative's name, representative's signature, date
B) Description of corrective action (CA)
Date of completion of corrective action
Representative's name, representative's signature, date
Is the corrective action above acceptable? Yes, no
If no, give reasons
Auditor's signature, date
C) Verification of corrective action
Acceptable documentation provided? Yes, no
Non-conformance corrected? Yes, no
Date of any completed follow-up visit (if required) and results
Can this Corrective Action Request be closed? Yes, no
If no, give reasons
Auditor's name, auditor's signature, date
Filing info
Appendix 3 - Sample Canadian Grain Sampling Program Notice of Suspension from the Canadian Grain Sampling Program
"Date"
"Manager name"
"Company name"
"Address"
Subject: Notice of Suspension from the Canadian Grain Sampling Program
Dear "manager name":
On "date" an audit of "company name" "(CGSP Identification number)", "location" was carried out by "auditor" from the Canadian Food Inspection Agency, "Office location". During this audit, serious non-conformances were found and are detailed in the attached audit report number (#).
While "company" is under suspension CFIA will no longer accept samples for phytosanitary analysis drawn by your company. Samples for phytosanitary analysis can only be taken by CFIA or an approved CGSP company. A list of CGSP approved companies can be found at "website".
If you wish to re-apply for participation under the CGSP you must re-submit a manual and complete an Application for Participation in the Canadian Grain Sampling Program. A detailed report of the corrective actions taken to address the previous non-conformances must also be included with the application. An evaluation audit will be conducted to determine the ability of your company to meet the requirements of the CGSP.
If you wish to discuss this further please contact "name" at "phone number".
Yours sincerely,
"RPO"
"Agency name"
cc "Auditor"
- Date modified: