CFIA procedure for organic equivalency determination and on-going monitoring of the existing arrangements
On this page
- 1. Scope and purpose
- 2. Authority
- 3. Requirements for equivalency application and determination
- 4. Equivalency arrangement implementation phase
- 5. Obligations of the parties
- Annexes
1. Scope and purpose
This procedure describes the approach to be followed by the Canadian Food Inspection Agency (CFIA) when negotiating an organic equivalency arrangement with a competent authority having jurisdiction in a foreign country and Canada's requirements for on-going monitoring of the existing arrangements.
2. Authority
The authority for the determination of a foreign country's equivalency status is provided by:
3. Requirements for equivalency application and determination
Overview
Equivalence is determined by assessing and comparing 2 regulatory systems to determine whether the principles and outcomes achieved are equivalent and negotiating an organic equivalence arrangement. The general process and components of an equivalence arrangement are:
- Equivalence request or application: A competent authority seeking an equivalence determination by Canada submits a request
- Acknowledgement and engagement: Upon receiving a request for an equivalence determination, Canada acknowledges receipt, verifies the request is complete, and determines whether it can proceed with evaluating the request
- Application review and assessment: If Canada determines that it can move forward with the equivalence request, it will begin the assessment process which typically includes document review and on-site audit(s)
- Technical discussions/negotiations: The 2 competent authorities engage in technical discussions to resolve differences identified in standards reviews and audits
- Determination: Following technical discussions, Canada makes its determination whether the requesting competent authority's system is equivalent or not and notifies the requesting country
- Signing of equivalence arrangement letters: The equivalence arrangement becomes effective once both parties sign arrangement letters determining each other's programs equivalent, or at a later date as agreed by both parties
- Ongoing monitoring of the arrangement: With the agreement, the parties establish an on-going monitoring process to verify the continued effectiveness of each other's control systems. The process typically includes annual reports and/or periodic peer reviews
3.1 Application phase
3.1.1 The foreign country is required to submit an application for equivalency determination in writing to the Senior Director Food Import and Export Division, International Program Directorate at the CFIA.
3.1.2 The request is made in the form of a written application package that includes the following information:
- The competent authority's contact person(s) and contact information.
- The legal basis for the foreign government technical measures and assessment system.
- Organizational chart/program structure – competent authority and others involved in implementation
- Scope of regulations
- Number of accredited certification bodies (CBs)
- Number of certified operations
- The scope of the requested determination (for example, agricultural products, livestock products, crop products).
- A detailed side-by-side comparison table of the foreign government's technical requirements and regulations completed in a provided table, along with a conformity assessment with detailed documentation explaining the foreign government's position on all differences.
- Supporting documentation describing all parts of the conformity assessment procedure used in the foreign country. This documentation should include legal authority, specifications and procedures, along with compliance and enforcement processes.
- All documents must be submitted in 1 of the official languages of Canada (English or French).
3.1.3 The CFIA will acknowledge receipt of the submission by official letter (hardcopy or digital copy) within 7 working days.
3.1.4 Application review and assessment
If the CFIA determines that it can move forward with the organic equivalence request, it will begin the assessment process which typically includes document review, on-site audit(s), and technical meetings with the requesting competent authority.
- Assessing the application: The first step for CFIA is to examine the application documentation for completeness. If the review identifies missing information, the CFIA notifies the applicant of the outstanding deficiencies that need to be addressed.
- Document review: The comparison analysis will be conducted in collaboration with other government departments and the industry. CFIA uses the application documents to conduct a side-by-side analysis of the requesting country's standards. This is a detailed comparison of the 2 systems showing alignment and differences. A side-by-side is submitted as part of the application using a pre-determined template developed by the CFIA. In addition, the CFIA may also complete its own side-by-side analysis. The CFIA will also conduct a document review of the other application materials that fall outside of the side-by-side.
- Report and clarification: When the side-by-side and document review activities are complete, a document review report is to be prepared and submitted to the applicant competent authority for discussion. Additional information or documents for clarification purposes might be requested to facilitate the comparison process. The 2 competent authorities may also hold meetings to discuss the report and clarify certain issues.
3.1.5 On-site audit and audit/assessment report
- On-site audit: Pending the outcome of the document review, the next step for the CFIA is to conduct 1 or more on-site audit(s) of the applicant competent authority. The objective of the on-site audit is to verify that the requesting competent authority's system is functioning as indicated in the side-by-side and document review.
- Audit/assessment report: Following the on-site audit an audit report is to be prepared and submitted by the CFIA to the applicant competent authority. The assessment report will be used to initiate the equivalence negotiation phase.
3.2 Equivalency negotiation phase
3.2.1 Once the on-site assessment report is complete, the 2 competent authorities will enter technical discussions, or the negotiation phase of the equivalence process. The objective of this phase is to work through the differences or issues identified during the document review and on-site audit(s), and to establish the scope and terms of an equivalence.
3.2.2 In formal negotiations, the CFIA will work with Global Affairs Canada (GAC) and Agriculture and Agri-Food Canada (AAFC) to determine the equivalency status.
3.2.3 The CFIA will work with Global Affairs Canada (GAC) and Agriculture and Agri-Food Canada (AAFC) to determine the scope of the negotiation, an agreement on the assessment process to be followed, and a potential timeline.
3.2.4 As a result of the document review and on-site audit(s), the CFIA may identify the aspects for which additional information is needed and any potential critical variances (for example, gaps or differences in standards).
3.2.5 The final step in negotiations is to agree on the terms of the recognition. This includes transforming any remaining critical variances into exceptions for the equivalence and to establish a mutually agreed upon monitoring system. The CFIA will deliver the results of equivalency status to the foreign country by either digital or hard copy delivery.
3.2.6 The CFIA in consultation with other government departments will prepare the letters or arrangement (Annex 1).
3.2.7 The equivalency agreement between the Canada and the foreign country will become effective upon signatures by both parties, or at later date as agreed by both parties.
3.2.8 The equivalency agreement may be amended at any time by mutual understanding of the parties.
3.2.9 The equivalency agreement may be terminated by either party upon 90 days written notice to the other party.
4. Equivalency arrangement implementation phase
4.1 The parties will establish an on-going monitoring process to verify the effectiveness of each other's control systems.
4.2 The process will include annual reports and/or peer reviews.
4.3 The annual report will cover the activities for the previous calendar year as specified in Annex 1 and is required to be submitted by March 31 of the current year (Annex 2).
4.4 Canada will review the annual report to determine on-going compliance with the existing arrangement and may request further information in support of the annual report.
4.5 Canada will issue notice of assessment as a result of the annual report review
4.6 Canada will establish an evaluation cycle which will identify the frequency of the peer reviews. The evaluation cycle may be adjusted based on risks and other factors.
4.7 The peer review will be conducted in accordance with the CFIA peer review procedure.
4.8 The parties may establish a technical working group (TWG) to discuss and provide guidance related to the equivalency arrangement. The parties will decide on the TWG terms of reference and the frequency of the TWG meetings.
5. Obligations of the parties
5.1 As a result of equivalency negotiation, obligations may vary in some respects depending on the circumstances of the particular determination.
5.2 Each party will notify the other of any planned legislative changes or revised equivalency determination procedures including changes in policies, procedures, standards or documents (certificates) which are relevant to the equivalency arrangement.
5.3 Each party shall promptly notify the other in writing of any changes in the list of its competent authorities and certification bodies (CB).
5.4 Each party shall promptly notify the other in writing of any instances that compromises the integrity of either party's organic certification program.
5.5 Each party will notify the other of any organic product certified under the terms of the equivalency arrangement -which is found to be in non-compliance, as a result of consumer or trade complaint.
5.6 Upon receipt of such a notification, the exporting country should undertake the necessary investigation to determine the cause of the problem.
Annexes
Annex 1 – Equivalency application and determination process
The Government of Canada including the CFIA oversee the equivalency application and determination process is, with support from the following partners:
- Federal Market Access Team
- Canadian Food Inspection Agency International (CFIA/International)
- Agriculture and Agri-Food Canada (AAFC)
- Global Affairs Canada (GAC)
- Post/Locally Engaged Staff (Post)
Equivalency application and determination process
Click on image for larger view
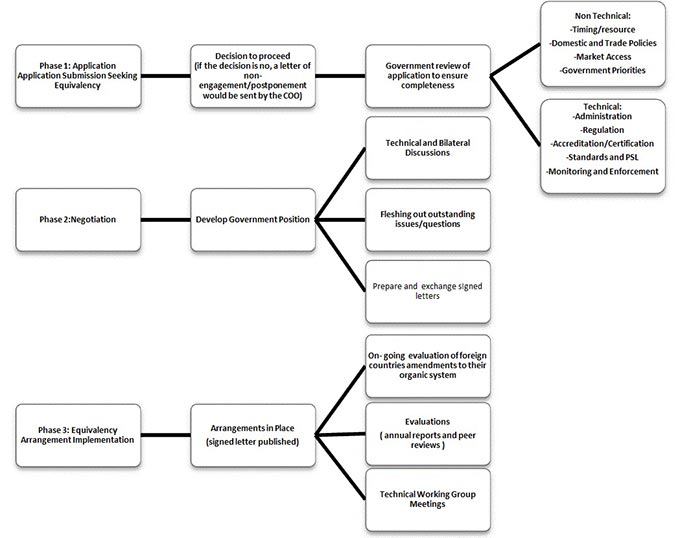
Description for figure - Equivalency application and determination process
Annex 1 Equivalency application and determination process
- This flow chart provides a supporting reference of how the Canada Organic Office Procedure for Equivalency Determination works. The process consists of three phases. Application, Negotiation, and Equivalency Arrangement Implementation.
Phase 1: Application
- Application submission seeking equivalency
- Decision to proceed (if the decision is no, a letter of non-engagement/postponement would be sent by the COO)
- Government review of application to ensure completeness
- Non Technical: Timing/resource, Domestic and Trade Policies, Market Access, and Government Priorities.
- Technical: Administration, Regulation, Accreditation/Certification, Standards and PSL, and Monitoring and Enforcement
Phase 2: Negotiation
- Develop Government Position
- Technical and Bilateral Discussions
- Fleshing out outstanding issues/questions
- Prepare and exchange signed letters
Phase 3: Equivalency Arrangement Implementation
- Arrangements in Place (signed letter published)
- On-going evaluation of foreign countries amendments to their organic system
- Evaluations (annual reports and peer reviews)
- Technical Working Group Meetings
Annex 2 – Annual report template
As part of the on-going monitoring of existing equivalence arrangements the foreign country is required to provide the following information covering the previous calendar year by March 31 of the current year:
- Types and quantities of organic certified organic products exported to Canada under the equivalency arrangement.
- Any amendments to the production standards and the regulatory system including the accreditation and certification procedures that have been adopted. Please describe and explain why the organic system remains equivalent to the Canada Organic Regime.
- A current list and complete contact information of all accreditation bodies and certification bodies recognized under the arrangement.
- Overview of the monitoring and supervisory activities carried out by the competent authorities, including analysis of reports and any other information transmitted by the accreditation bodies and certification bodies.
- Overview of the enforcement action taken by the competent authorities. Please provide information on complaints management, follow up on positive residue testing and cancellation of organic certification.
- Any other relevant information such as follow-up on complaints under the equivalence arrangement of results from working group discussions.
- Date modified: