Honey Bee Producer Guide to the National Bee Farm-level Biosecurity Standard
Appendix D: Diagnosis and monitoring methods: Main pests affecting honey bees
This page is part of the Guidance Document Repository (GDR).
Looking for related documents?
Search for related documents in the Guidance Document Repository
American foulbrood
Regular inspections of the brood area of honey bee colonies are advised, especially during the active spring, summer, and fall. Visually examine brood frames for signs of American Foulbrood (AFB). Signs include an unhealthy, scattered brood pattern with sunken/punctured cappings that appear dark or greasy. Diseased brood combs also have a characteristic fish-like odour. Any discoloured brood should be examined closely, under good lighting, and at a slight angle so that the lower sides of the cell can be seen clearly. Infected larvae settle to the bottom or the side of the cell wall in a sunken gooey mass, which is beige to dark brown in colour. Occasionally, the tongue of a dead pupa is found extending and attaching to the cell wall. Brood frames should be carefully examined for scale (hardened dark black masses of old dead larvae) that adhere directly to the cell wall.
Detect AFB using the ropiness test – insert a twig, matchstick, or toothpick into the cell to stir larva, and draw out the contents. A 2 cm rope with the consistency of mucous will be stretched from the stick if it is AFB. AFB can also be confirmed through microscopic examination or molecular testing. The spores resemble slender rods in chains. Contact your bee regulating authority if you are unsure of your AFB diagnosis or if you find AFB .
European foulbrood
Regular inspections of the brood area are advised, especially in spring and early summer. European Foulbrood (EFB) is often associated with susceptibility factors. Signs include an unhealthy brood pattern with capped and uncapped cells being found scattered irregularly over the frame, and twisted pearly white coloured larvae in a C shape in the cells. Larvae infected with EFB typically die before cell capping and give off an offensive, sour smell. In some cases, infected larvae may generate little or no odour.
EFB -infected larva turn yellow at first and then brown, at which time the tracheal system becomes visible as a glistening vein-like network throughout the larval body. The larvae eventually decay to a point where they form a dry, rubbery scale in the cell; the scale can be removed quite easily by the bees.
Prior to forming a dry scale, the larvae become somewhat softened and granular. Test for ropiness, using a twig, matchstick, or toothpick that is inserted into the larvae, and stir. Then, draw out the content, but it will not reveal the typical gluey long string or ropy signs of AFB , but rather a dry appearance.
Advanced microbiological culture and molecular tests are also available. Contact your provincial apiarist or bee authority for more information.
Chalkbrood
Regular inspections of the brood area are advised. Signs of infection can include patchy irregular brood, and white and/or black mummies in cells. An early sign of chalkbrood infection is small pinhole-sized holes chewed into the cappings by the worker bees. Eventually, worker bees will uncap the cells and remove the mummies, discarding them at the hive entrance and/or on the bottom board. Pollen traps (under the hive) should also be checked for chalkbrood mummies. Monitoring for chalkbrood should be increased during cold spring and high moisture climates.
Sacbrood
Regular inspections of the brood area are advised, especially in spring and early summer, when forage is limited. Signs include patchy brood, punctured cell cappings, and larvae that look like a watery sac. Some cells may remain capped after the surrounding brood has emerged. Diseased brood usually die in the pre-pupal stage. They tend to darken prematurely from white to yellow, and finally change to a dark brown colour once they die. Dead larvae will be found lying along the length of a cell with a slightly raised darkened head. If left long enough, the larval remains will dry out and settle into a brittle scale that can easily be removed from the cell.
Nosema disease
Nosema is caused by 2 microsporidian (fungal) parasites: Nosema apis; more recently, Nosema ceranae. It is the most widespread disease of adult honey bees in Canada. Nosema apis typically has the highest levels in the spring, whereas Nosema ceranae may also be detected throughout the season. Signs of generalized Nosema disease include gradual depopulation, slow population buildup in spring and summer, and higher winter losses. Nosema apis infection can include dysentery, crawling behaviour in front if the hive, disjointed wings, distended abdomens, and the loss of the sting reflex. Dysentery and crawling bees are not commonly seen with Nosema ceranae infections.
Signs of Nosema may not be visually evident, especially if infestations are light. Behavioural signs are similar to those of pesticide poisoning, and poor quality food stores may cause dysentery. Therefore, confirming Nosema spore presence and determining spore counts are necessary to determine whether recommended treatment thresholds have been reached.
Ideally, microscopic and molecular tests should be performed by a diagnostic laboratory with beekeepers who have been properly trained in the sampling, slide preparation, and identification procedures.
Alternatively, follow provincial apiarist, bee authority, or laboratory-recommended procedures when collecting and preparing samples to be sent for analysis and confirmation of diagnosis. Typically 30 to 60 adult bees are collected from the front entrance or inner cover of a suspected colony. Selecting older foraging bees provides a more reliable result, as younger housekeeping bees are less likely to be infected with Nosema. For an apiary composite sample, collect approximately 10 bees from each colony (up to 10 colonies per apiary). Place sample in a 50 to 70% alcohol solution to preserve the bees. Bee samples may also be frozen or dried for later analysis. Label sample by colony number and apiary with the beekeepers name and contact information.
Honey bee tracheal mites
Honey bee tracheal mite (HBTM), Acarapis woodi, is an internal parasite. It lives and reproduces in the tracheal system of honey bees. They feed on bee hemolymph. The mites infect adult worker, drone, and queen honey bees, and can be serious if not treated. Tracheal mites affect the overwintering CAPA bility of bee colonies, cause disjointed wings (called K-wing) and result in infested bees crawling near the hives. A heavy HBTM load diminishes brood area, reduces bee populations, results in low honey yields and, ultimately, in colony loss.
Collect samples in early spring or early fall for monitoring tracheal mites. To determine the efficacy of your treatment: collect samples in the fall, if you treat in the spring, or vice versa.
There are 2 sampling methods:
- Individual bee colony samples: Collect 50 to 75 bees per hive from honey combs or the inner cover. Place collected bees in a jar containing 70% alcohol. From each apiary, collect samples from 6 hives. In each operation, collect samples from 5 to 10 apiaries. Then, dissect 30 to 50 bees per colony. Check the infestation levels under the microscope to determine the average mite prevalence
- Composite bee sample representing an apiary: Collect 5 to 10 bees from honey combs or the inner cover from each hive in an apiary of 25 to 40 colonies. Place collected bees in a jar containing 70% alcohol. Collect composite samples from 5 to 10 apiaries. Dissect only 150 bees for each apiary, and examine the tracheae for the presence of tracheal mites under a microscope.
Varroa mite
The Varroa mite (Varroa destructor) is an external parasite that feeds on adults, larvae, and pupae, and is visible to the unaided eye; visual signs include shrivelled wings on emerging bees, patchy brood pattern, and reddish mites on bees or comb. Because Varroa is relatively common, monitoring is used to determine mite count thresholds that may trigger treatment or to evaluate treatment efficacy (resistance), and not only confirm presence in the colony.
Several monitoring methods are available:
1. Quantitative determination of treatment threshold levels
-
(24-Hour) Sticky board: Quick natural mite drop on a sticky trap
Pros: The natural mite drop is a reliable method to determine treatment threshold counts if Varroa presence is confirmed. A sticky board also prevents mites from climbing and reinfesting bees.
Con: The natural mite drop requires a repeat visit to the apiary. In addition, leaving the sticky boards for more than 72 hours makes it difficult to count the mites, due too much debris.
Method:
- Coat a thick piece of paper (filing folders [38 X 30 cm ] work well), using 50% Vaseline/50% Crisco, Tangle Trap paste, or Sticky Stuff
- Place the coated paper under a screen, and on the bottom board for 3 days
-
Count the Varroa adult mites on the sticky board, and divide by 3 to obtain an average mite fall per day
Natural Varroa mite drop is affected by a number of factors, including weather and genetics. For this reason, monitor over 3 days, calculating the average per day to use in reference to treatment thresholds
- Varroa hand shaker
Pros: Simple, reliable, and fast method to carry out in the field and does not require a second trip.
Con: Bees in the samples are killed during the process:
Methods:
- Collect approximately 300 worker bees (1/3 cup) from brood frames into a sample jar that contains (up to half of the jar) winter windshield washing fluid, or 70% alcohol. A total of 300 dead bees will fill about 1 inch (25 mm) in the bottom of the jar
- Screw the sample jar onto the hand shaker, and then shake the Varroa hand shaker vigorously up, down, and sideways for 40 to 60 seconds
- Turn the jar with the bees upside down to keep the bees on the top of the screen, allowing the mites in liquid to pass through into the bottom jar
- Check and count the number of mites collected in the fluid in the bottom jar
-
To determine the percent infestation, use the following equation: multiply the number of counted mites by 1.3. This will give you the corrected number of mites. Then, divide the corrected number of mites by 3. For example, assuming that you collected 300 bees, and counted 7 mites in the bottom jar, the total number of mites is equal to 7 X 1.3 = 9. The percentage infestation is equal to 9 divided by 3 = 3%
Handheld, easy to use, commercially made mite shaker devices that give effective and fast results are also available. Follow the directions given with the shaking apparatus. Contact your local bee supply outlet for availability
- Honey bee diagnostic lab
Collect the recommended number of bees, as indicated by the provincial apiarist, bee authority, or laboratory, and place in a jar of alcohol, and ship to a honey bee diagnostic lab.
2. Qualitative indicators of varroa presence
Pros: This method can be carried out in one visit to the field. Confirmation of Varroa presence may flag the need for sticky board method.
Con: It is not as accurate as quantitative methods for determining whether treatment thresholds have been reached.
-
Ether roll
Ether roll is a quick field test. Place 125 ml (1/2 cup) of bees (approximately 300 bees) from the brood chamber in a glass jar. Spray with 3 or 4 squirts of ether (engine starter fluid). Replace lid and shake for one full minute. Roll the jar, and then count the Varroa stuck to the glass and under the lid. Do this in a well-ventilated area, wearing gloves to minimize contact with the ether
-
Sugar roll (dusting)
Sugar roll is a quick field test. Use a double jar, with perforated lids bonded together. Scoop 125 ml (1/2 cup) of bees (approximately 300 bees) from the brood chamber, and place in one jar. Add 30 ml (2 tablespoons) of powdered (icing) sugar to the other jar, shaking gently back and forth to coat the bees. Empty the coated bees onto a flat surface, away from the wind, and count the Varroa mites remaining in the sugar as the bees walk away
-
Uncapping drone brood
This method can be done off-site with frozen samples at the beekeepers' convenience. First, record the amount of drone brood per colony. Uncap and remove larvae/pupae from 100 drone cells, recording the number that are infested with Varroa mites. Count both immature and (adult) mature mites. Compare the level of infestation to the total amount of drone brood in the colony
3. Threshold guidelines for varroa
Once results are obtained, consult the treatment thresholds that are indicated in the annually updated provincial recommendations for mite control. If your province does not publish annual recommendations, contact your provincial department. Treatment methods are established for each monitoring method and for 2 periods: early May and late August. Treatment threshold levels may be lower if more than one pest is present (for example, Varroa and tracheal mites).
If treatments were applied and Varroa infestation is at the threshold level or higher the next spring and there were abnormally high losses over the winter, it is advised to test for acaricide resistance.
In June, monitor all bee yards (at least 5 hives in each), using the monitoring method of choice to determine whether Varroa mites were controlled by the spring treatment.
Monitor a number of bee yards to represent your operation. In each bee yard, examine at least 5 hives in early August, using the monitoring method of your choice to ensure that Varroa infestations are not at, or above, treatment threshold levels. If they are highly infested, they will have sustained enough damage that the colony will not winter properly and will more likely die. Treat early fall or as early as possible after removing honey supers to protect winter bees. If honey supers are still on and mite levels are high, remove honey supers and treat immediately. Treatments that are applied late in September and October have reduced efficacy, compared with treatments applied earlier.
Due to potential efficacy constraints caused by environmental conditions with treatments that are temperature dependent and have potential for resistance in chemical treatments, it is highly recommended that monitoring occur after treatments to determine whether the treatment was effective or whether there is a need for a follow-up fall treatment (after brood production stops). Late nectar flows and inclement weather can delay the treatment of colonies in the fall, resulting in reduced efficacy of mite control products and high winter kill.
The monitoring process should therefore include monitoring and recording of environmental conditions.
Varroa can have several generations per year and thus can develop resistance to some treatments within a short time. For this reason, beekeepers should frequently monitor for suspected resistance.
Small hive beetle
Egg, larval, and adult stages of the small hive beetle (SHB) and damage caused by the larvae of SHB can be observed in the honey bee colony, on exposed hive equipment, or in packaged material of imported bees.
Inspect hives for the presence of the small hive beetle. Examine the tops of brood frames (particularly towards the ends of the frames) for the presence of SHB adults immediately after the lid is removed, because adult beetles will run away from light. Adult and larval beetles may also be encountered on the bottom board or on the surface of brood frames among worker bees. Inspect for SHB adults on the bottom board by quickly looking for the adult beetles as soon as the brood chamber is tipped to expose the bottom board.
Extraction facilities should also be monitored for the presence of SHB.
A variety of mechanical traps may be used to monitor for SHB. Consult bee-supply outlets for a range of options, or contact your provincial apiarist or bee authority for recommendations.
The adult beetle is about 3/16" (5.5 mm) long, 1/8" (3.2 mm) wide, and dark brown in colour. It has clubbed antennae. The larva looks similar to a wax moth larva, but the SHB larva has spines along the length of its body. The SHB larva has 3 pairs of true legs, while the wax moth caterpillar has 3 pairs of true legs plus prolegs (false legs). SHB larva does not spin a cocoon in the hive. Adult and larval SHB can also be found in dead colonies and exposed colony equipment.
If SHB is suspected in your hives, immediately contact your provincial apiarist or honey bee regulating authority. Follow all notification procedures, and permit and quarantine regulations.
Monitoring for SHB includes ongoing awareness of confirmed presence in your local area.
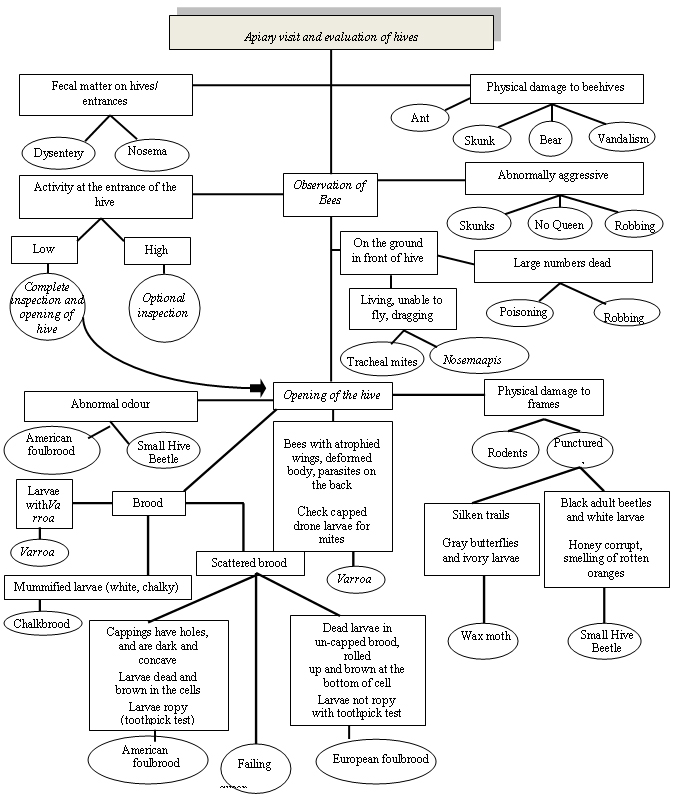
Figure 1 describes the possible diagnosis of honey bee pests related to monitoring techniques. An evaluation of hives occurs during apiary visits. If fecal matter is present on hives or entrances this may be a sign of Dysentery or Nosema. Physical damage to beehives may be caused by ants, skunks, bears, or vandalism. During observation of the bees if activity at the entrance of the hive is low, a complete inspection (opening) of the hive should be conducted. This inspection is optional if activity is high. Abnormally aggressive bees may be the result of skunks, robbing or the absence of a queen. A large number of dead bees on the ground in front of the hive may be attributed to poisoning or robbing. If a large number of living bees (unable to fly or dragging) is observed on the ground in front of the hive, tracheal mites or Nosema apis may be the cause. An abnormal odour inside the hive may indicate the presence of American foulbrood or Small Hive Beetle. Physical damage to the frames could be the result of rodents or punctured combs. Puncutred combs with silken trails, gray moths, and ivory larvae result from Wax moths. Small Hive Beetle results in punctured combs with white larvae and black adult beetles and honey that smells of rotten oranges. The presence of Varroa may result in bees with atrophied wings, deformed bodies, and parasites on the backs of the bees. Chalkbrood causes mummified larvae that are white and chalky. Scattered brood may be the result of American foulbrood, European foulbrood, or a failing queen. American foulbrood causes ropy dead larvae, brown coloured cells, and cappings with holes that are concave in shape. European foulbrood causes dead brown larvae usually found rolled up at the bottom of un-capped cells however the larvae are not ropy.
Description for flowchart - Figure 1 Honey bee pest diagnosis and monitoring
Figure 1 is titled Honey Bee Pest Diagnosis and Monitoring. This figure is a flow chart outlining apiary observations and possible causes.
- The first box is labelled: Apiary visit and evaluation of hives. A line leads to a box labelled:
- Observation of Bees
- From Observation of Bees six lines lead to six boxes labelled:
- Fecal matter on hives/entrances. From here two lines lead to two circles labelled:
- Dysentery
- Nosema
- Physical damage to beehives. From here four lines lead to four circles labelled:
- Ant
- Skunk
- Bear
- Vandalism
- Activity at the entrance of the hive. From here two lines lead to two boxes labelled:
- Low. From here a line leads to a circle labelled
- Complete inspection and opening of hive. From here an arrow leads to a box labelled
- Opening of the hive
- Complete inspection and opening of hive. From here an arrow leads to a box labelled
- High. From here a line leads to a circle labelled
- Optional inspection
- Low. From here a line leads to a circle labelled
- Abnormally Aggressive. From here three lines lead to three circles labelled:
- Skunks
- No Queen
- Robbing
- On the ground in front of the hive. From here two lines lead to two boxes labelled:
- Living, unable to fly, dragging. From here two lines lead to two circles labelled:
- Tracheal mite
- Nosema apis
- Large numbers dead. From here two lines lead to two circles labelled:
- Poisoning
- Robbing
- Living, unable to fly, dragging. From here two lines lead to two circles labelled:
- Fecal matter on hives/entrances. From here two lines lead to two circles labelled:
- From Observation of Bees a line leads to a box labelled: Opening of the hive. From here four lines lead to four boxes labelled:
- Abnormal odour. From here two lines lead to two circles labelled:
- American foulbrood
- Small Hive Beetle
- Brood. From here three lines lead to three boxes labelled:
- Larvae with Varroa. From here a line leads to a circle labelled Varroa
- Mummified larvae (white, chalky). From here a line leads to a circle labelled
- Chalkbrood
- Scattered brood. From here three lines lead to two boxes and one circle labelled:
- The first box is labelled Cappings have holes, and are dark and concave. Larvae dead and brown in the cells. Larvae ropy (toothpick test). From here a line leads to a circle labelled
- American foulbrood
- The circle is labelled Failing queen
- The second box is labelled Dead larvae in un-capped brood, rolled up and brown at the bottom of cell. Larvae not ropy with toothpick test. From here a line leads to a circle labelled
- European foulbrood
- The first box is labelled Cappings have holes, and are dark and concave. Larvae dead and brown in the cells. Larvae ropy (toothpick test). From here a line leads to a circle labelled
- Bees with atrophied wings, deformed body, parasites on the back. Check capped drone larvae for mites. From here a line leads to a circle labelled:
- Varroa
- Physical damage to frames. From here two lines lead to two circles labelled:
- Rodents
- Punctured. From here two lines lead to two boxes labelled:
- Silken trails. Gray butterflies and ivory larvae. From here a line leads to a circle labelled
- Wax Moth
- Black adult beetles and white larvae. Honey corrupt, smelling of rotten oranges. From here a line leads to a circle labelled:
- Small Hive Beetle
- Silken trails. Gray butterflies and ivory larvae. From here a line leads to a circle labelled
- Abnormal odour. From here two lines lead to two circles labelled:
- Date modified: